trans-4-Butylcyclohexanecarboxylic acid | 38289-28-0
中文名称
——
中文别名
——
英文名称
trans-4-Butylcyclohexanecarboxylic acid
英文别名
trans-4-n-butylcyclohexanecarboxylic acid;trans-e,e-4-butylcyclohexanecarboxylic acid;trans-4-n-butyl-cyclohexanecarboxylic acid;4-butyl-trans-cyclohexanecarboxylic acid;trans 4-butylcyclohexanecarboxylic acid
CAS
38289-28-0
化学式
C11H20O2
mdl
——
分子量
184.279
InChiKey
BALGERHMIXFENA-MGCOHNPYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37°C
-
沸点:287.7±8.0 °C(Predicted)
-
密度:0.973±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
稳定性/保质期:
常规情况下不会分解,也没有危险反应。
计算性质
-
辛醇/水分配系数(LogP):3.07
-
重原子数:13.0
-
可旋转键数:4.0
-
环数:1.0
-
sp3杂化的碳原子比例:0.91
-
拓扑面积:37.3
-
氢给体数:1.0
-
氢受体数:1.0
安全信息
-
危险等级:IRRITANT
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2916209090
-
储存条件:密封、阴凉、干燥保存。
SDS
trans-4-Butylcyclohexanecarboxylic Acid Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: trans-4-Butylcyclohexanecarboxylic Acid
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): trans-4-Butylcyclohexanecarboxylic Acid
Percent: >99.0%(GC)(T)
CAS Number: 38289-28-0
Chemical Formula: C11H20O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
trans-4-Butylcyclohexanecarboxylic Acid
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Almost white
Odor: No data available
pH: No data available
Melting point/freezing point:37 °C
Boiling Point/Range: No data available
Flash Point: No data available
trans-4-Butylcyclohexanecarboxylic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
trans-4-Butylcyclohexanecarboxylic Acid
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: trans-4-Butylcyclohexanecarboxylic Acid
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): trans-4-Butylcyclohexanecarboxylic Acid
Percent: >99.0%(GC)(T)
CAS Number: 38289-28-0
Chemical Formula: C11H20O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
trans-4-Butylcyclohexanecarboxylic Acid
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Almost white
Odor: No data available
pH: No data available
Melting point/freezing point:37 °C
Boiling Point/Range: No data available
Flash Point: No data available
trans-4-Butylcyclohexanecarboxylic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: Soluble in : Methanol
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
trans-4-Butylcyclohexanecarboxylic Acid
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对丁基环已烷甲酸 4-butylcyclohexanecarboxylic acid 71101-89-8 C11H20O2 184.279 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— Cyclohexanecarboxylic acid, 4-pentyl-, cis- 38289-29-1 C12H22O2 198.305 —— trans-4-Hexylcyclohexanecarboxylic acid 38289-30-4 C13H24O2 212.332 —— (4-Butyl-cyclohexyl)-acetic acid 71458-19-0 C12H22O2 198.305
反应信息
-
作为反应物:描述:trans-4-Butylcyclohexanecarboxylic acid 在 lithium aluminium tetrahydride 、 氯化亚砜 、 硫酸 、 氢溴酸 作用下, 以 乙醚 、 溶剂黄146 、 二甲基亚砜 为溶剂, 生成 (4-Butyl-cyclohexyl)-acetyl chloride参考文献:名称:Carr; Gray; McDonnell, Molecular Crystals and Liquid Crystals (1969-1991), 1982, vol. 97, # 1-4, p. 13 - 28摘要:DOI:
-
作为产物:描述:参考文献:名称:Liang, Jason C.; Cross, Julie O., Molecular Crystals and Liquid Crystals (1969-1991), 1986, vol. 133, p. 235 - 244摘要:DOI:
文献信息
-
Iron-Catalyzed Vinylic C−H Alkylation with Alkyl Peroxides作者:Liang Ge、Wujun Jian、Huan Zhou、Shaowei Chen、Changqing Ye、Fei Yu、Bo Qian、Yajun Li、Hongli BaoDOI:10.1002/asia.201800534日期:2018.9.4are readily accessible from carboxylic acids, are utilized as general primary, secondary, and tertiary alkylating reagents for iron‐catalyzed vinylic C−H alkylation of vinyl arenes, dienes, and 1,3‐enynes. This transformation affords olefinic products in up to 98 % yield with high E/Z values. A broad range of functionalities, including carboxyl, boronic acid, methoxy, ester, amino, and halides, are
-
N-(Cyclohexylcarbonyl)-D-phenylalanines and related compounds. A new class of oral hypoglycemic agents. 2作者:Hisashi Shinkai、Masahiko Nishikawa、Yusuke Sato、Koji Toi、Izumi Kumashiro、Yoshiko Seto、Mariko Fukuma、Katsuaki Dan、Shigeshi ToyoshimaDOI:10.1021/jm00127a006日期:1989.7the decarboxyl derivative, the esters, and the amides of the phenylalanine derivatives. Thus, the structural requirements for possessing hypoglycemic activity was elucidated and a highly active compound, N-[(trans-4-isopropylcyclohexyl)carbonyl]-D-phenylalanine (13) was obtained, which showed a 20% blood glucose decrease at an oral dose of 1.6 mg/kg in fasted normal mice.
-
Synthesis and Thermal and Photoluminescence Properties of Liquid Crystalline Polyacetylenes Containing 4-Alkanyloxyphenyl <i>trans</i>-4-Alkylcyclohexanoate Side Groups作者:Ching-Hua Ting、Jiun-Tai Chen、Chain-Shu HsuDOI:10.1021/ma0107962日期:2002.2.1Two series of polyacetylenes containing 4-alkanyloxyphenyl trans-4-n-alkylcyclohexanoate were synthesized using [Rh(nbd)Cl]2, MoCl5, and WCl6 as initiators. Polymers 1P−3P, which contain no flexible spacer, show no mesomorphic properties due to the rigid poly(phenylacetylene) backbone. Polymers 4P−9P, which contain three or four methylene units in their spacers, exhibit both SA and Sc phases. X-ray
-
New (1R,4R)-2-arylidene-p-menthan-3-ones with a bridging ester group in the arylidene fragment. Synthesis and behavior in liquid-crystalline systems作者:V. V. Vashchenko、L. A. Kutulya、M. N. Pivnenko、N. I. ShkolnikovaDOI:10.1023/b:rucb.0000012363.37580.30日期:2003.11(1R,4R)-2-(4-Hydroxybenzylidene)- and (1R,4R)-2-(4'-hydroxybiphenyl-4-yl)methylene-p-menthan-3-ones were synthesized by condensation of (-)-menthone with O-tetrahydropyran-2-yl derivatives of 4-hydroxybenzaldehyde and 4'-hydroxy-4-formylbiphenyl, respectively, in a DMSO-base medium followed by the removal of the protective group. The reactions of these hydroxy derivatives with 4-alkylbenzoic, 4-alkyloxybenzoic, traps-4-alkylcyclohexane-4-carboxylic, and 4'-alkylbiphenyl-4-carboxylic acids afforded three series of new chiral esters. Compounds containing the arylidene moiety with three benzene rings were found to exhibit liquid-crystalline properties. The characteristic features of these compounds are discussed based on the results of studies by polarizing microscopy, differential scanning calorimetry, and small-angle X-ray scattering. It was found that the mesomorphic compounds under study can form a smectic A mesophase, twist grain boundary mesophases (TGBA), and blue phases in a wide temperature range. Upon dissolution of certain of chiral compounds in 4'-cyano-4-pentylbiphenyl, a rather high twisting power and the thermal stabilizing effect on mesophases were observed.(1R,4R)-2-(4-羟基苯甲基)-和(1R,4R)-2-(4'-羟基二苯基-4-基)甲基-p-薄荷烷-3-酮是通过(-)-薄荷酮与4-羟基苯甲醛和4'-羟基-4-甲酰二苯基的O-四氢吡喃-2-基衍生物在DMSO基介质中缩合,随后去除保护基团而合成的。这些酚衍生物与4-烷基苯甲酸、4-烷氧基苯甲酸、陷阱-4-烷基环己烷-4-羧酸以及4'-烷基二苯基-4-羧酸的反应生成了三类新的手性酯。含有三苯环肉桂基结构的这些衍生物被发现具有液晶性质。基于偏光显微镜、差示扫描量热法和小角X射线散射法的研究结果,讨论了这些化合物的特征。发现研究中的介晶化合物可以在较宽的温度范围内形成一个层状A介晶相、扭曲晶界介晶相(TGBA)以及蓝相。当某些手性化合物溶解于4'-氰基-4-戊基二苯基中时,观察到较高的扭曲力和对介晶相的热稳定效应。
-
Long-acting androgenic compounds and pharmaceutical compositions thereof申请人:——公开号:US04948790A1公开(公告)日:1990-08-14Long-acting androgenic compositions comprising C.sub.4 -C.sub.6 cycloalkyl carboxylic acids of testosterone and a pharmaceutically acceptable carrier medium. The ester can be dissolved in an acceptable oily medium or suspended in an aqueous medium in the form of micronised particles or in the form of milled particles.
表征谱图
-
氢谱1HNMR
-
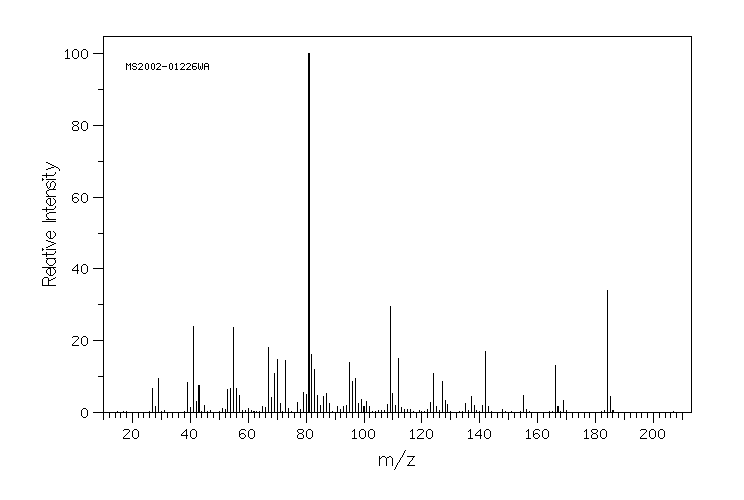
质谱MS
-
碳谱13CNMR
-
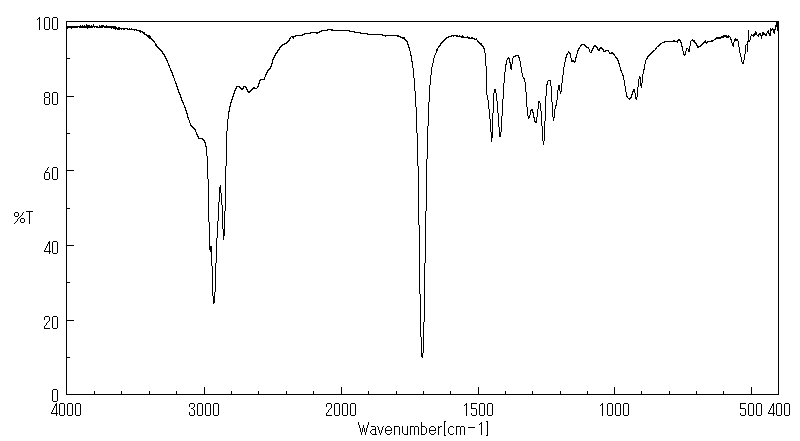
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸