(E)-4-secbutylbut-3-en-2-one | 875587-77-2
中文名称
——
中文别名
——
英文名称
(E)-4-secbutylbut-3-en-2-one
英文别名
5-methyl-3(E)-hepten-2-one;(E)-5-methylhept-3-en-2-one;5-methyl-hept-3-en-2-one;5-methyl-3-hepten-2-one
CAS
875587-77-2
化学式
C8H14O
mdl
——
分子量
126.199
InChiKey
QDUQNQQMHZNNTG-AATRIKPKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:173.8±9.0 °C(Predicted)
-
密度:0.831±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.62
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
反应信息
-
作为反应物:描述:(E)-4-secbutylbut-3-en-2-one 、 苯肼,盐酸盐 在 氧气 、 sodium acetate 、 oxovanadium(IV) sulfate 作用下, 以 水 、 甲苯 为溶剂, 反应 20.0h, 以58%的产率得到5-sec-butyl-3-methyl-1-phenyl-1H-pyrazole参考文献:名称:Synthesis of Functionalized Pyrazoles via Vanadium-Catalyzed C–N Dehydrogenative Cross-Coupling and Fluorescence Switch-On Sensing of BSA Protein摘要:Vanadium-catalyzed C-N dehydrogenative cross-coupling of alkenyl hydrazones leading to functionalized pyrazoles is described in a 1:1 mixture of toluene/H2O using air as the terminal oxidant. Significant practical features include use of the commercial nontoxic VOSO4 as a recyclable catalyst, mild reaction conditions, scalability, and the broad substrate scope. Some of the product pyrazoles exhibit interesting photophysical properties. Fluorescence light-up sensing of BSA protein by one of the pyrazoles is also highlighted.DOI:10.1021/acs.orglett.5b02669
-
作为产物:参考文献:名称:钯催化烯丙基α-烯基-β-酮酸酯的还原性脱羧。(E)-3-烯酮的新合成摘要:烯丙基-β-酮酸酯的α-烯基衍生物的还原脱羧反应是通过使用由Pd(OAc)2和PPh 3原位生成的钯(0)催化剂,以甲酸三乙铵为氢化物源,在THF中实现的。该反应可与烯丙基-β-酮酸酯的线性烯基衍生物平稳且清洁地进行,以良好至极佳的收率(73–92%)和高的立体选择性(> 98%)提供(E)-3-烯酮。DOI:10.1016/j.tetlet.2005.11.122
文献信息
-
[EN] INDAZOLES<br/>[FR] INDAZOLES申请人:GLAXOSMITHKLINE LLC公开号:WO2011140325A1公开(公告)日:2011-11-10Herein are disclosed indazoles of formula (I) where the various groups are defined herein, and which are useful for treating cancer.以下披露了式(I)的吲唑化合物,其中各种基团在此处定义,并且这些化合物对治疗癌症有用。
-
Morpholinium Trifluoroacetate-Catalyzed Aldol Condensation of Acetone with both Aromatic and Aliphatic Aldehydes作者:Kristina Zumbansen、Arno Döhring、Benjamin ListDOI:10.1002/adsc.200900902日期:——We report a highly efficient, general and practical method for the aldol condensation of acetone with aromatic and aliphatic aldehydes, using morpholinium trifluoroacetate as a catalyst.
-
3,6-Dimethyl-3-hydroxy-oct-1-ynes and -oct-1-enes, derivatives of these,申请人:BASF Aktiengesellschaft公开号:US04347388A1公开(公告)日:1982-08-313,6-Dimethyl-3-hydroxy-oct-1-ynes and -oct-1-enes and their esters with lower alkanoic acids, of the general formula I ##STR1## where X and Y are H or the two X's and/or the two Y's together are a further bond between the carbon atoms on which they are present, and R is H, --CO--CH.sub.3, --CO--C.sub.2 H.sub.5 or --CO--C.sub.3 H.sub.7, their use as scents, and a process for the preparation of 3,6-dimethyl-3-hydroxy-octane. The novel compounds exhibit interesting, predominantly floral, woody, herbal and fruity notes. The alcohols of the formula I are of particular importance because they serve as intermediates for a novel and particularly advantageous method of obtaining 3,6-dimethyl-3-hydroxy-octane, a compound required in large amounts as a fragrance for soaps and detergents.
-
Synthesis of <i>E</i>-α,β-Unsaturated Ketones with Complete Stereoselectivity via Sequential Aldol-Type/Elimination Reactions Promoted by Samarium Diiodide or Chromium Dichloride作者:José Concellón、Humberto Rodríguez-Solla、Carmen Concellón、Pamela DíazDOI:10.1055/s-2006-933146日期:——E-α,β-Unsaturated ketones can be obtained with complete E-selectivity by a sequential reaction of dichloroketones with a variety of aldehydes. This transformation was promoted by SmI2, SmI2 in the presence of FeCl3 or CrCl2. The best results were obtained when CrCl2 was used as the metallating agent. A mechanism based on a successive aldol-type reaction and a β-elimination is proposed to explain these results.
-
BICYCLIC HETEROCYCLIC COMPOUND申请人:Saito Tetsuji公开号:US20100137318A1公开(公告)日:2010-06-03A compound of a formula (I): wherein R 1 represents a C3-10 branched alkyl group which may be substituted; R 2 represents a hydrogen atom or a C1-4 alkyl group which may be substituted; R 3 represents a C1-4 alkyl group which may be substituted or a halogen atom; R 4 represents a C1-4 alkyl group which may be substituted; and ring 1 represents a cyclic group which has planarity and may have a substituent group, a salt thereof, an N-oxide thereof or a solvate thereof, or a prodrug thereof, is useful as a medicinal component having CRF antagonistic activity for the prevention and/or treatment of a neuropsychiatric disease, a peripheral organ disease and the like.化合物的化学式(I):其中R1代表一个C3-10支链烷基,可以被取代; R2代表氢原子或C1-4烷基,可以被取代; R3代表C1-4烷基,可以被取代或卤素原子; R4代表C1-4烷基,可以被取代; 环1表示具有平面性且可能具有取代基团的环状基团,其盐,其N-氧化物或其溶剂化物,或其前药,可用作具有CRF拮抗活性的药物成分,用于预防和/或治疗神经精神疾病,外周器官疾病等。
表征谱图
-
氢谱1HNMR
-
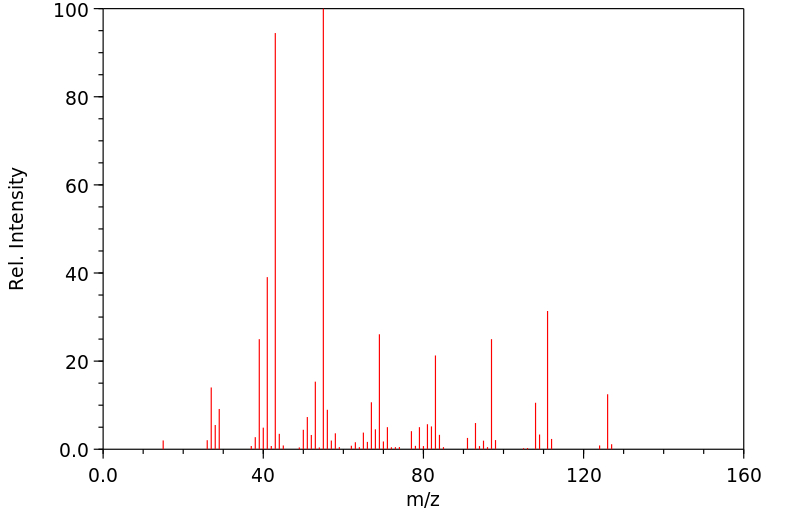
质谱MS
-
碳谱13CNMR
-
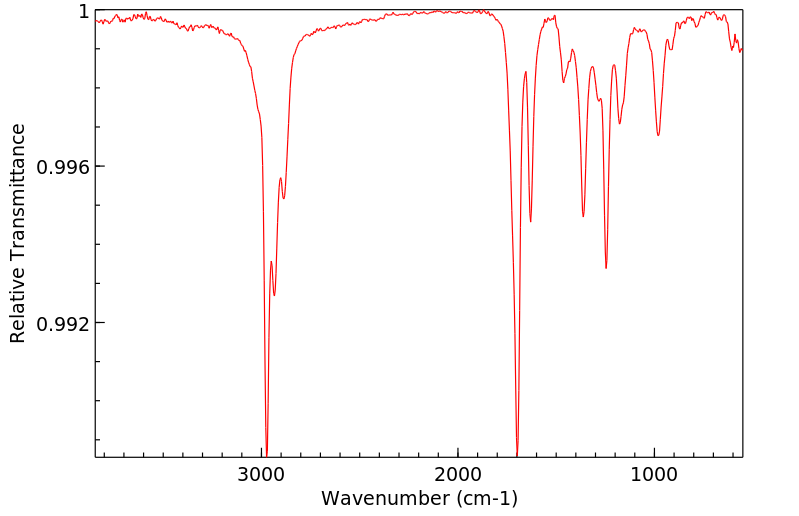
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷