3-乙酰苯磺酰氯氟化物 | 709-60-4
中文名称
3-乙酰苯磺酰氯氟化物
中文别名
3-乙酰苯磺酰氟
英文名称
3-acetylbenzenesulfonyl fluoride
英文别名
3-Fluorsulfonyl-acetophenon
CAS
709-60-4
化学式
C8H7FO3S
mdl
MFCD00007416
分子量
202.206
InChiKey
NEVFJPDNDDMQGD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:59.6
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
海关编码:2914700090
SDS
| Name: | 3-Acetylbenzenesulfonyl fluoride 99% Material Safety Data Sheet |
| Synonym: | 3-acetylbenzenesulfonyl fluoride; m-(Fluorosulfonyl)acetophenon |
| CAS: | 709-60-4 |
Synonym:3-acetylbenzenesulfonyl fluoride; m-(Fluorosulfonyl)acetophenon
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 709-60-4 | 3-Acetylbenzenesulfonyl fluoride | 99 | 211-912-5 |
Risk Phrases: 34 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Causes burns. Irritating to eyes, respiratory system and skin.Moisture sensitive.Corrosive.
Potential Health Effects
Eye:
Causes eye burns.
Skin:
Causes skin burns.
Ingestion:
The toxicological properties of this substance have not been fully investigated. Causes digestive tract irritation with possible burns.
Inhalation:
The toxicological properties of this substance have not been fully investigated. Causes respiratory tract irritation with possible burns.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Discard contaminated clothing in a manner which limits further exposure. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Do NOT use water directly on fire. Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Discard contaminated shoes.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 709-60-4: United States OSHA: 2.5 mg/m3 TWA (as F) (listed under Fluorides Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: tan
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 90.00 - 92.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: CH3COC6H4SO2F
Molecular Weight: 202.20
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, moisture.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide, fluoride fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 709-60-4: DB8941000 LD50/LC50:
Not available.
Carcinogenicity:
3-Acetylbenzenesulfonyl fluoride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE SOLID, N.O.S.*
Hazard Class: 8
UN Number: 1759
Packing Group: II
IMO
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE SOLID, N.O.S.
Hazard Class: 8
UN Number: 1759
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI C
Risk Phrases:
R 34 Causes burns.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 709-60-4: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 709-60-4 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 709-60-4 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-acetyl-N,N-dimethylbenzenesulfonamide 173158-15-1 C10H13NO3S 227.284
反应信息
-
作为反应物:描述:3-乙酰苯磺酰氯氟化物 、 二乙胺 在 二氯甲烷 、 水 、 magnesium sulfate 作用下, 以 四氢呋喃 为溶剂, 反应 88.0h, 以to give 45 g (88%) of thick oil的产率得到3-(N,N-diethylaminosulfonyl)acetophenone参考文献:名称:Renin inhibitors摘要:该文献介绍了一种公式为:##STR1##其中A是从以下公式中选择的残基:##STR2##其中Z为O、S、SO或SO.sub.2,P为1或2,X为--O--或--S--;以及抑制肾素底物裂解作用的类似物,包含这些化合物的制药组合物,制备这些化合物的方法以及使用这些新型肾素抑制剂治疗高血压的方法。公开号:US05459131A1
文献信息
-
Novel tricyclic compounds and drug compositions containing same申请人:ASAHI KASEI KOGYO KABUSHIKI KAISHA公开号:US20030139475A1公开(公告)日:2003-07-24Compounds having a &bgr;-3 adrenaline receptor agonist and are useful as drugs for the treatment and prevention of diabetes, obesity, hyperlipemia, etc., represented by a general formula (I) and salts thereof, and a process for producing these, and their intermediates, wherein R represents hydrogen or methyl; R 1 represents hydrogen, halogen, hydroxy, benzyloxy, amino, or hydroxymethyl; R 2 represents hydrogen, hydroxymethyl, NHR 3 , SO 2 NR 4 R 4′ , or nitro; R 6 represents hydrogen or lower alkyl; and X represents nitrogen, R 9 represents hydrogen, one of R 7 and R 8 represent hydrogen, and the other thereof represents hydrogen, amino, acetylamino, or hydroxy.
-
Process for the preparation of an amine terminated polybutadiene申请人:ELF ATOCHEM S.A.公开号:EP0206714A1公开(公告)日:1986-12-30@ Novel amine terminated polybutadiene compounds of the formula: wherein R is hydrogen, a straight or branched chain alkyl group containing from 1 to 10 carbon atoms or a substituted or unsubstituted aryl or aralkyl group containing one or more benzenoid rings which may be fused or joined by single valency bonds, and n is an integer of from about 5 to 1500 and a method for the preparation thereof by reacting a polyhydroxybutadiene homopolymer with an alkane- or arenesulfonyl chloride or fluoride in the presence of a tertiary amine to form an alkane- or arenesulfonate terminated polybutadiene and then reacting said alkane- or arenesulfonate terminated polybutadiene with a primary amine or ammonia.
-
NOVEL TRICYCLIC COMPOUNDS AND DRUG COMPOSITIONS CONTAINING THE SAME申请人:Asahi Kasei Kogyo Kabushiki Kaisha公开号:EP0882707A1公开(公告)日:1998-12-09Compounds represented by general formula (I) or salts thereof, a process for producing the same, and intermediates therefor, wherein R represents hydrogen or methyl; R1 represents hydrogen, hologeno, hydroxy, benzyloxy, amino, or hydroxymethyl; R2 represents hydrogen, hydroxymethyl, NHR3, SO2NR4R4, or nitro; R6 represents hydrogen or lower alkyl; and X represents nitrogen, oxygen, sulfur, or methylene, provided that when X represents nitrogen, oxygen, or sulfur, then R9 represents hydrogen, one of R7 an R8 represents hydrogen, and the other thereof represents hydrogen, amino, acetylamino, or hydroxy, and when X represents methylene, then R7 and R8 each represents hydrogen and R9 represents hydrogen, amino, etc. They have a β-3 adrenaline receptor agonist and are useful as drugs for the treatment and prevention of diabetes, obesity, hyperlipemia, etc.
-
NOVEL TRICYCLIC COMPOUNDS HAVING SATURATED RINGS AND MEDICINAL COMPOSITIONS CONTAINING THE SAME申请人:Asahi Kasei Kogyo Kabushiki Kaisha公开号:EP0997458A1公开(公告)日:2000-05-03Compounds represented by general formula (I) or their salts, having β3 -adrenoceptor agonism and being efficacious when employed in drugs for treating and preventing diabetes, obesity, hyperlipemia, etc. wherein R represents hydrogen or methyl; R1 represents hydrogen, halogeno, hydroxy, benzyloxy, amino or hydroxymethyl; R2 represents hydrogen, hydroxymethyl, NHR3, SO2NR4R4', or nitro (wherein R3 represents hydrogen, methyl, SO2R5, formyl or CONHR6'; R5 represents lower alkyl, benzyl or NR4R4'; R4 and R4' may be the same or different and each represents hydrogen, lower alkyl or benzyl; and R6' represents hydrogen or lower alkyl); R6 represents hydrogen or lower alkyl; n is 1 or 2; X represents secondary nitrogen, oxygen or sulfur; and when n is 1, then one of R7 and R8 represents hydrogen while another represents hydrogen, amino, acetylamino or hydroxy, or when n is 2, then R8 represents hydrogen while R7 represents hydrogen, amino, acetylamino or hydroxy.通式(I)所代表的化合物或其盐类,具有 β3-肾上腺素受体激动作用,在用于治疗和预防糖尿病、肥胖症、高血脂症等药物时具有疗效。其中 R 代表氢或甲基;R1 代表氢、卤素、羟基、苄氧基、氨基或羟甲基;R2 代表氢、羟甲基、NHR3、SO2NR4R4'或硝基(其中 R3 代表氢、甲基、SO2R5、甲酰基或 CONHR6';R5 代表低级烷基、苄基或 NR4R4';R4 和 R4'可以相同或不同,各自代表氢、低级烷基或苄基;和 R6' 代表氢或低级烷基);R6 代表氢或低级烷基;n 为 1 或 2;X 代表仲氮、氧或硫;当 n 为 1 时,则 R7 和 R8 中的一个代表氢,而另一个代表氢、氨基、乙酰氨基或羟基,或当 n 为 2 时,则 R8 代表氢,而 R7 代表氢、氨基、乙酰氨基或羟基。
-
Method, composition and device for sampling natriuretic peptides in a biological fluid申请人:Scios Inc.公开号:EP2098866A1公开(公告)日:2009-09-09Disclosed is a composition that synergistically prevents proteolysis or modification of peptides in sampled biological fluids using sulfonyl fluoride family protease inhibitors at high concentrations combined with at least one additional protease inhibitor of a different type, preferably a broad spectrum protease inhibitor, and a chelator. A preferred embodiment uses AEBSF at 10mM, Benzamidine at 20mM and EDTA as the chelator. The disclosed composition may be combined with other protease inhibitors to further modulate its specificity, for instance to additionally target acidic proteases. Additional protease inhibitors, reducing agents, stabilizers and buffering agents may be combined with the disclosed compositions in devices for sampling or testing biological fluids for levels of peptides of interest, or methods therefore. The disclosed devices, compositions and methods are of particular use in sampling and testing for the level of natriuretic peptides.
表征谱图
-
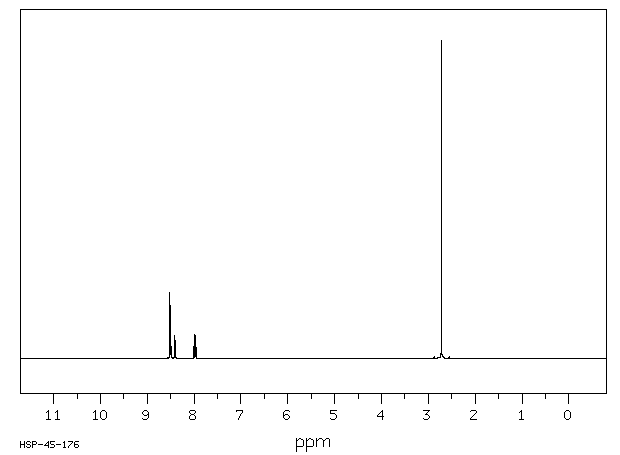
氢谱1HNMR
-
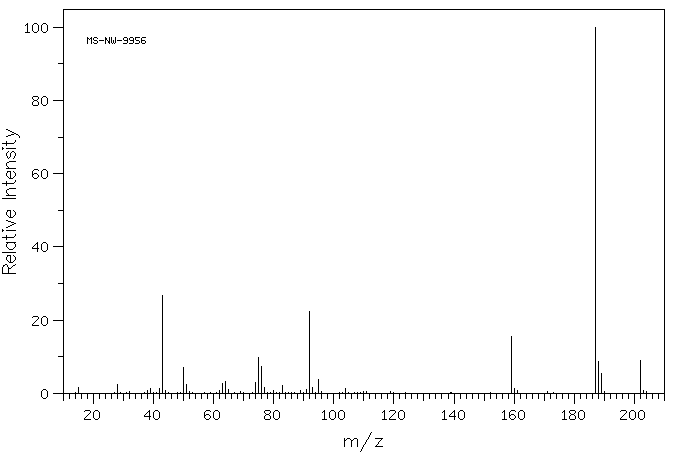
质谱MS
-
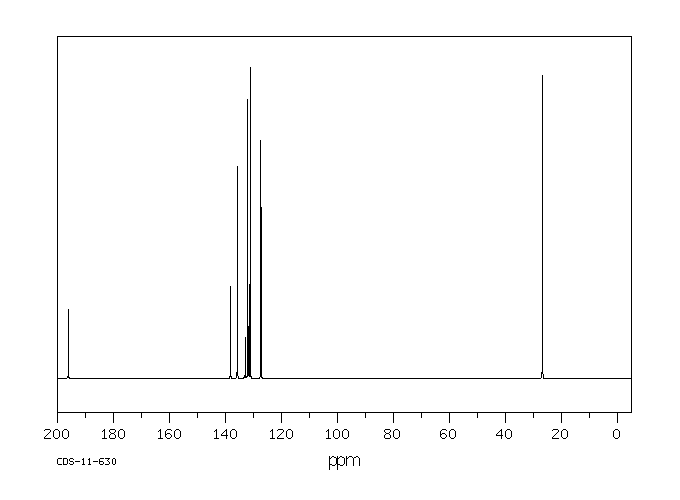
碳谱13CNMR
-
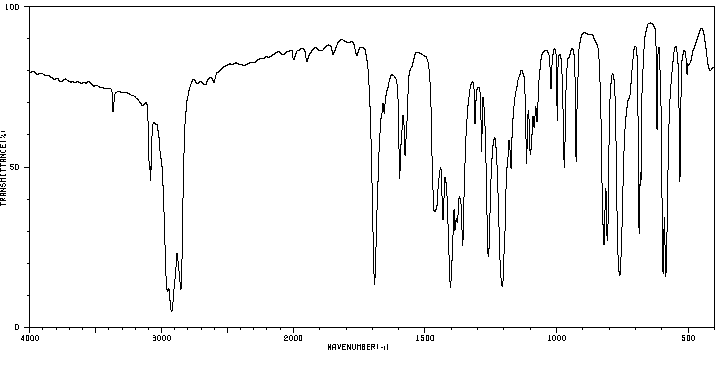
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷