3-乙基环戊酮 | 10264-55-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:150 °C
-
沸点:171℃
-
密度:0.9015 g/cm3(Temp: 425 °C)
-
闪点:31°(88°F)
-
LogP:1.220 (est)
-
保留指数:965;975;967;923;923;959
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3
-
安全说明:S16
-
危险类别码:R10
-
海关编码:2914299000
-
包装等级:III
-
危险品运输编号:UN 1224
SDS
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:3-乙基环戊酮 在 H2SO4 作用下, 生成 4-ethyl-2-piperidinone参考文献:名称:Amidino dervatives useful as nitric oxide synthase inhibitors摘要:本发明公开了含有用作一氧化氮合酶抑制剂的氮杂环衍生物的有用药物组合物。公开号:US05854234A1
-
作为产物:描述:参考文献:名称:Rabiller,C. et al., Bulletin de la Societe Chimique de France, 1974, p. 3055 - 3058摘要:DOI:
文献信息
-
Efficient Palladium(0) supported on reduced graphene oxide for selective oxidation of olefins using graphene oxide as a ‘solid weak acid’作者:Xi Gao、Jianhao Zhou、Xinhua PengDOI:10.1016/j.catcom.2019.01.020日期:2019.3Selective oxidation of olefin derivatives to ketones has made innovative development over palladium(0) supported on reduced graphene oxide. Compared to traditional Wacker oxidation, the novel method offers an economical and environment-friendly option by using graphene oxide (GO) as a ‘solid weak acid’ instead of classical homogeneous catalysts like H2SO4 and CF3COOH. X-ray diffraction, X-ray photoelectron
-
Competing Radical- and Anion-Mediated Pathways in the Reduction of Bridgehead Tosylates with Lithium Aluminium Hydride作者:Ernest W. Della、Wit K. JanowskiDOI:10.1071/ch98165日期:——
Reaction of norborn-1-yl tosylate with lithium aluminium hydride in boiling tetrahydrofuran affords a mixture of norbornan-1-ol accompanied by the ring-opened products 4-methylcyclohexanol and 3-ethylcyclopentanol as their cis/trans isomers, as well as p-thiocresol and p-tolyl disulfide. Evidence strongly suggests that the reaction is mediated by the norborn-1-yloxy radical rather than the norborn-1-yloxy anion. The process is initiated by very slow acyl oxygen fission of the norbornyl tosylate, followed by reduction of the derived p-toluenesulfinate ion to give the p-thiocresoxide anion. Transfer of an electron from the latter to the substrate and decomposition of the derived norborn-1-yl tosylate radical anion leads to the norborn-1-yloxy radical which, upon ring opening, generates the monocyclic alcohols via the corresponding ketones. It is noteworthy that, when norborn-1-yl mesylate is exposed to lithium aluminium hydride, it yields norbornan-1-ol exclusively. In the absence of an efficient electron-transfer agent, the mechanism of reaction of norborn-1-yl mesylate is suggested to involve acyl oxygen fission only.
在沸腾的四氢呋喃中,降冰片烷-1-基甲苯磺酸盐与氢化铝锂发生反应 在沸腾的四氢呋喃中与氢化铝锂反应,生成降冰片烷-1-醇的混合物,同时生成 4-甲基环己醇和 3-乙基环戊醇的开环产物。 开环产物 4-甲基环己醇和 3-乙基环戊醇的混合物。 顺/反异构体,以及 对硫代甲酚和对甲苯基 二硫化物。证据有力地表明,该反应是由 而不是降冰片-1-氧基阴离子。该过程 是由非常缓慢的酰氧基裂解降冰片酰基对甲苯磺酸盐开始的,然后由衍生的对甲苯磺酸盐还原 生成的对甲苯磺酸离子被还原,生成对硫代甲磺酸阴离子。 生成对硫代甲磺酸阴离子。电子 电子转移到底物上,并分解衍生出的降冰片-1-基 基阴离子分解,产生降冰片-1-基氧基基,该基在开环后生成单环化合物。 开环后,通过相应的酮生成单环醇。值得注意的是 值得注意的是,当降冰片-1-基甲磺酸酯与铝氢化锂接触时 氢化锂时,只生成降冰片烷-1-醇。如果没有有效的 在缺乏有效电子转移剂的情况下,降冰片-1-基甲磺酸酯的反应机理被认为涉及酰基氧裂变。 建议只涉及酰基氧裂变。 -
Substituted Imidazopyridines as HDM2 Inhibitors申请人:Merck Sharp & Dohme Corp.公开号:US20140179680A1公开(公告)日:2014-06-26The present invention provides substituted imidazopyridines as described herein or a pharmaceutically acceptable salt or solvate thereof. The representative compounds are useful as inhibitors of the HDM2 protein. Also disclosed are pharmaceutical compositions comprising the above compounds and potential methods of treating cancer using the same.
-
Anti-infective agents申请人:——公开号:US20040087577A1公开(公告)日:2004-05-06Compounds having the formula 1 are hepatitis C (HCV) polymerase inhibitors. Also disclosed are a composition and method for inhibiting hepatitis C (HCV) polymerase, processes for making the compounds, and synthetic intermediates employed in the processes.具有公式1的化合物是丙型肝炎(HCV)聚合酶抑制剂。还公开了一种用于抑制丙型肝炎(HCV)聚合酶的组成和方法,用于制造这些化合物的过程,以及在这些过程中使用的合成中间体。
-
Suzuki–Miyaura Coupling of Simple Ketones via Activation of Unstrained Carbon–Carbon Bonds作者:Ying Xia、Jianchun Wang、Guangbin DongDOI:10.1021/jacs.8b02462日期:2018.4.25Here, we describe that simple ketones can be efficiently employed as electrophiles in Suzuki-Miyaura coupling reactions via catalytic activation of unstrained C-C bonds. A range of common ketones, such as cyclopentanones, acetophenones, acetone and 1-indanones, could be directly coupled with various arylboronates in high site-selectivity, which offers a distinct entry to more functionalized aromatic
表征谱图
-
氢谱1HNMR
-
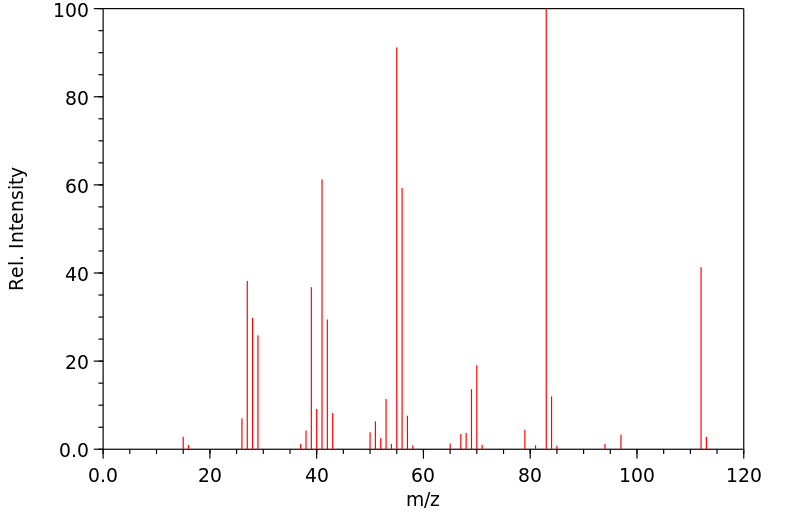
质谱MS
-
碳谱13CNMR
-
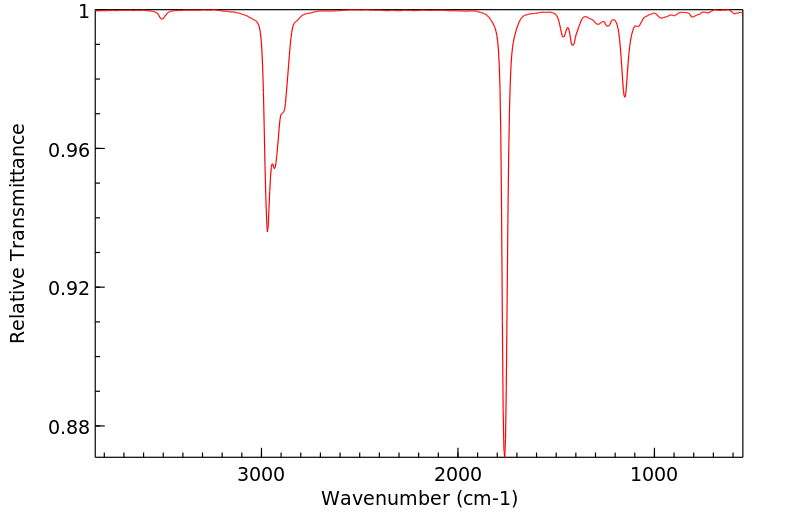
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息