3,3,6,6-四甲氧基-1,4-环己二烯 | 15791-03-4
中文名称
3,3,6,6-四甲氧基-1,4-环己二烯
中文别名
——
英文名称
1,1,4,4-tetramethoxycyclohexa-2,5-diene
英文别名
3,3,6,6-tetramethoxy-1,4-cyclohexadiene;3,3,6,6-tetramethoxycyclohexa-1,4-diene
CAS
15791-03-4
化学式
C10H16O4
mdl
MFCD00010207
分子量
200.235
InChiKey
LJAWVXAZDYVFNU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:45-48 °C(lit.)
-
沸点:60 °C/0.2 mmHg(lit.)
-
密度:1.1508 (rough estimate)
-
闪点:106 °C
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:36.9
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:8
-
危险品标志:C
-
危险类别码:R34
-
危险品运输编号:UN 1759
-
海关编码:2914509090
-
包装等级:III
-
危险类别:8
-
安全说明:S26,S27,S28,S36/37/39,S45
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1,4,4-Tetraethoxy-1,4-dihydrobenzene 72205-84-6 C14H24O4 256.342 —— 1,4-benzoquinone bis(ethylene ketal) 35357-33-6 C10H12O4 196.203 4,4-二甲氧基-2,5-环己二烯-1-酮 4,4-dimethoxycyclohexa-2,5-dienone 935-50-2 C8H10O3 154.166
反应信息
-
作为反应物:描述:参考文献:名称:rubiginones A(2)和C(2)及其11-甲氧基区域异构体的不对称合成。摘要:安古环素类天然产物茜草酮A(2)(2)和C(2)(1)及其11-甲氧基区域异构体3a和3b的融合对映选择性合成是通过使用两个对映体纯的1的多米诺过程实现的-乙烯基环己烯4.合成该二烯的关键步骤是在(SS)-[[(对甲苯基亚磺酰基)甲基]-对-喹诺酚(9)上立体选择性共轭加成AlMe(3)和消除β-羟基亚砜片段,氧化成砜后,回收羰基。第一多米诺序列包括与亚磺酰基萘醌的Diels-Alder反应,然后消除亚砜。在四烯骨架的收敛构造中,使用二烯亲二物5或6的C-2或C-3处的亚砜,在环加成步骤中实现了有效的相对区域选择。衍生自5-甲氧基-1,4-萘醌。第二个多米诺骨牌工艺是由氧气和阳光触发的,它使最初的四环加合物在B环芳构化,甲硅烷基脱保护和C-1氧化后转化为最终产物。DOI:10.1002/chem.200600809
-
作为产物:描述:参考文献:名称:Hydrogenation of Benzene Ring by Paired Electrosynthesis with Raney-Nickel Cathode摘要:以 1,4-二甲氧基苯 (1) 为底物进行了配对电解研究。在雷尼-镍阴极上,芳香环发生氢化反应,生成 1,4-环己二酮(4)和两种衍生物。通过盐酸水溶液的处理,两种衍生物转化成了 4。在 1 的基础上,4 的总产率为 60%,而在铂或铅阴极上则不会生成 4。DOI:10.1246/cl.1987.1989
文献信息
-
Anodic cleavage of carbon–oxygen bonds in diaryl ethers作者:Paulo R. Janissek、Vera L. Pardini、Hans ViertlerDOI:10.1039/c39870000576日期:——Diaryl ethers (1), which are model compounds for important linkages in lignins, are cleaved efficiently and conveniently by constant current anodic oxidation in methanol; the bis-acetals (2) are the main products (in 50–80% yield).
-
Studies of chromenes. Part 9. Syntheses of chromenequinones作者:Philip E. Brown、Robert A. Lewis、Mark A. WaringDOI:10.1039/p19900002979日期:——Syntheses of 2,2-dimethylchromene-5,8-quinone, 2,2-dimethylchromene-6,7-quinone, and 6,7-dimethoxy-2,2-dimethylchromene-5,8-quinone are described. Being easily bioreducible to electron-rich chromenes these compounds, and their oxirane derivatives, are of interest as possible anti-tumour alkylating agents.
-
Arenium cation as the key intermediate of the electrosynthesis of N-(2,5-dimethoxyphenyl)azoles. A new approach to the synthesis of N-(dimethoxyphenyl)azoles作者:V. A. Petrosyan、A. V. BurasovDOI:10.1007/s11172-007-0342-3日期:2007.11Data on the effect of the acid-base properties of the medium on the yield and composition of the products of N-dimethoxyphenylation of azoles (pyrazole, triazole, their substituted derivatives, and tetrazole) upon galvanostatic electrolysis of azole—1,4-dimethoxybenzene mixtures in nucleophilic (MeOH) and neutral (MeCN) media were considered and the trends of this process were discussed. The generation of arenium cations (1,4-dimethoxy-1-azolylbenzenium in MeCN and 1,1,4-trimethoxybenzenium in MeOH) as the key intermediates of electrosynthesis of N-(dimethoxyphenyl)azoles, was proved experimentally. A new approach to the synthesis of N-(dimethoxyphenyl)azoles through electrosynthesis of 1,1,4,4-tetramethoxycyclohexa-2,5-diene by electrooxidation of 1,4-dimethoxybenzene in MeOH as the first step and the reaction of this quinone diketal with azoles as the second step was suggested. The efficiency of this route to N-(dimethoxyphenyl)azoles is comparable with the efficiency of the purely electrochemical one-step process.
-
Synthesis of Azobenzenes from Quinone Acetals and Arylhydrazines作者:M. Carmen Carreño、Gerardo Fernández Mudarra、Estíbaliz Merino、María RibagordaDOI:10.1021/jo0498011日期:2004.5.1Direct reaction between quinone bisacetals and arylhydrazines gives azobenzenes. The presence of catalytic amounts of cerium ammonium nitrate strongly accelerates the reaction. When the bisacetal has a substituent at the 2,5-cyclohexadiene framework, only one regioisomer is formed. The method represents a simple, mild, and novel synthetic access to differently substituted azocompounds in high to excellent
-
Experiments on the total synthesis of Lysolipin I. Part II.Michael addition of 1,3-cyclohexanedione to quinone acetals作者:Rudolf O. Duthaler、Urs H.-U. WegmannDOI:10.1002/hlca.19840670713日期:1984.11.7Base-catalyzed reaction of 1,3-cyclohexanedione (3) with the quinone monoacetals 4 and 7 leads to the polycyclic products 5 and 8, respectively, and in the case of 4 to variable amounts of dibenzofuranone 6. The 2-arylcyclohexanedione 9, on the other hand, is isolated from the reaction of 3 and bisacetal 11 catalyzed by ZnCl2 (Scheme 2). Treatment of the adduct 8 with (CH3O)2SO2/K2CO3 results in cleavage1,3-环己二酮(3)与醌单缩醛4和7的碱催化反应分别产生多环产物5和8,在4的情况下产生可变量的二苯并呋喃酮6。另一方面,从由ZnCl 2催化的3与双缩醛11的反应中分离出2-芳基环己二酮9(方案2)。用(CH 3 O)2 SO 2 / K 2 CO 3处理加合物8结果通过逆迈克尔反应裂解杂环,得到可靠的烯酮23,该烯酮通过选择性氢化进一步转化为24。8-乙酰氧基二苯并呋喃酮22可通过酸处理和乙酰化从方案8获得(方案4)。甲硅烷基醚27和35与醌单缩醛的反应非常复杂(方案6)。所需的芳基环己酮衍生物28和36以非常低的产率形成。在某些条件下(高温或强路易斯酸作为催化剂),单电子转移或加到烯缩醛而不是醌单缩醛的烯酮功能上变得占主导地位。结合该研究,已经制备并表征了敏感的2-甲氧基对对苯醌单缩醛15(方案3)和29(方案6)。
表征谱图
-
氢谱1HNMR
-
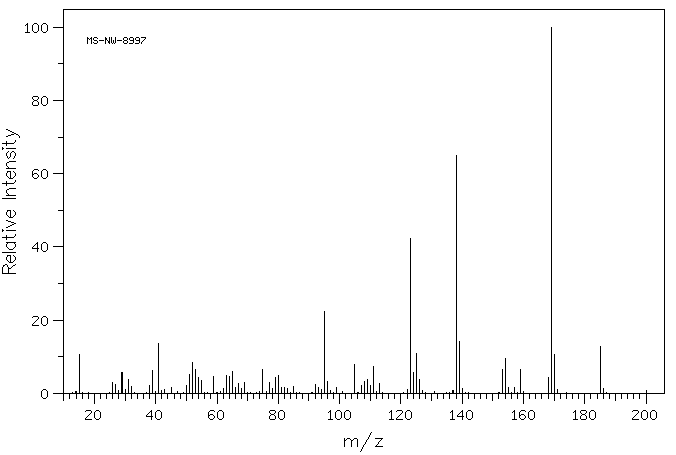
质谱MS
-
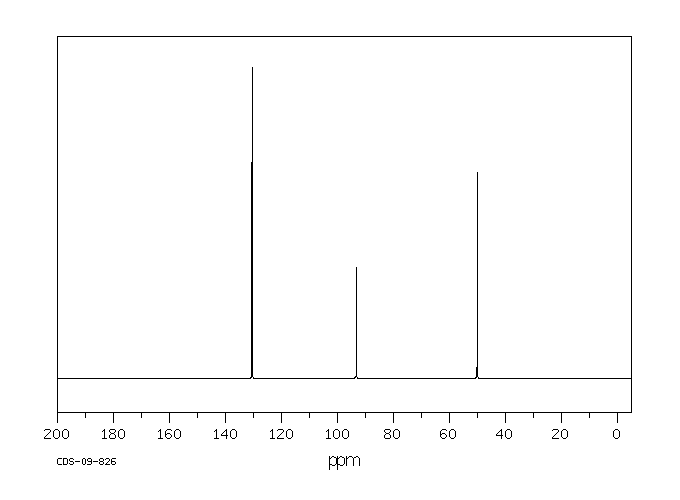
碳谱13CNMR
-
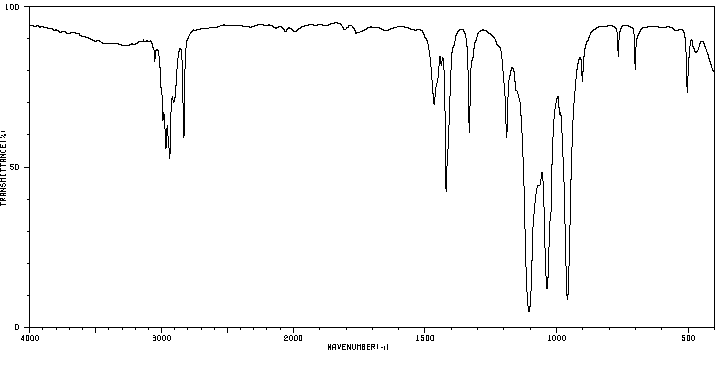
红外IR
-
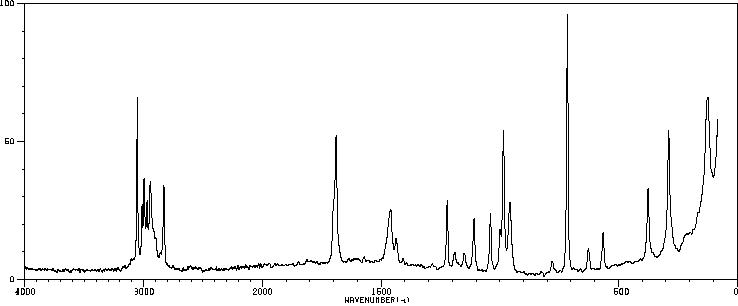
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷