3-乙基-3-庚醇 | 19780-41-7
中文名称
3-乙基-3-庚醇
中文别名
——
英文名称
3-ethylheptan-3-ol
英文别名
3-ethyl-3-heptanol;Diaethyl-butyl-carbinol;3-Heptanol, 3-ethyl-
CAS
19780-41-7
化学式
C9H20O
mdl
——
分子量
144.257
InChiKey
XKRZDNKKANUBPV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6.15°C (estimate)
-
沸点:192.9°C (estimate)
-
密度:0.8370
-
保留指数:853
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:10
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2905199090
SDS
反应信息
-
作为反应物:参考文献:名称:铁催化剂上醇和酮的有效气相脱氧摘要:讨论了一种在600 K和1-2·10 5 Pa下铁催化剂上将醇和酮气相脱氧为烃的方法。DOI:10.1016/s0040-4039(00)98301-1
-
作为产物:参考文献:名称:Jur'ew et al., Zhurnal Obshchei Khimii, 1959, vol. 29, p. 3652,3654; engl. Ausg. S. 3611摘要:DOI:
文献信息
-
Oxalic Acid-catalyzed Reaction of Alcohols with NaSCN: The Effects of Additives NaI and I<sub>2</sub>作者:Hideyoshi Miyake、Yuichi Nakao、Mitsuru SasakiDOI:10.1246/cl.2006.1262日期:2006.11Oxalic acid-mediated conversion of alcohols to thiocyante and/or isothiocyanate is described. Aliphatic tertiary alcohols give isothiocyanate by the reaction with NaSCN in the presence of I2, whereas they give thiocyanate without it.
-
Direct transformation of dialkyl sulfates into alkyllithium reagents by a naphthalene-catalysed lithiation作者:David Guijarro、Gabricia Guillena、Balbino Mancheño、Miguel YusDOI:10.1016/s0040-4020(01)87022-8日期:1994.3The lithiation of primary and secondary dialkyl sulfates with an excess of lithium powder in the presence of a catalytic amount of naphthalene (4 mol %) in THF at −78°C leads to the corresponding alkyllithium reagents (1:2 molar ratio) which react with different electrophiles, mainly carbonyl compounds, to yield after hydrolysis, the expected coupling products. This methodology represents an indirect
-
Process for Production of Alkyl Tin Alkoxide Compound, and Process for Production of Carbonic Acid Ester Using the Compound申请人:Shinohata Masaaki公开号:US20100292496A1公开(公告)日:2010-11-18The present invention provides a process for producing: a compound represented by XOR 2 ; a dialkyl tin dialkoxide compound having one tin atom, two Sn—R 1 bonds and two Sn—OR 2 bonds; and/or a tetraalkyl dialkoxy distannoxane compound having one Sn—O—Sn bond, in which each tin atom of the tetraalkyl dialkoxy distannoxane compound has two Sn—R 1 bonds and one Sn—OR 2 bond, the process comprising reacting in the absence of a catalyst at least one alkyl tin compound selected from the group consisting of i) and ii) below: i) a dialkyl tin compound having one tin atom, two Sn—R 1 (wherein R 1 represents an alkyl group) bonds, and two Sn—OX bonds (wherein OX is a group in which HOX that is a conjugate acid of OX is a Bronsted acid having a pKa of from 0 to 6.8); and ii) a tetraalkyl distannoxane compound having one Sn—O—Sn bond, in which each tin atom of the tetraalkyl distannoxane compound has two Sn—R 1 bonds and one Sn—OX bond (wherein OX is a group in which HOX that is a conjugate acid of OX is a Bronsted acid having a pKa of from 0 to 6.8); and a carbonic acid ester represented by R 2 OCOOR 2 (wherein R 2 represents a linear or branched, saturated or unsaturated hydrocarbon group, a hydrocarbon group having a saturated or unsaturated cyclic hydrocarbon substituent, or a Y—CH 2 — group (wherein Y represents an alkyl polyalkylene group, an aromatic group or a cyclic saturated or unsaturated alkylene ether group)), and/or an alcohol represented by R 2 OH (wherein R 2 is the same as defined above).本发明提供了一种生产过程:产生一个由XOR表示的化合物;具有一个锡原子、两个Sn—R1键和两个Sn—OR2键的二烷基锡二烷氧化合物;和/或具有一个Sn—O—Sn键的四烷基二烷氧基二锡烷氧化合物,其中四烷基二烷氧基二锡烷氧化合物的每个锡原子具有两个Sn—R1键和一个Sn—OR2键,所述过程包括在缺乏催化剂的情况下反应以下所述组中选择的至少一种烷基锡化合物: i) 具有一个锡原子、两个Sn—R1(其中R1代表烷基基团)键和两个Sn—OX键(其中OX是HOX的共轭酸,HOX是具有从0到6.8的pKa的Bronsted酸的群)的二烷基锡化合物;和 ii) 具有一个Sn—O—Sn键的四烷基二锡烷氧化合物,其中四烷基二锡烷氧化合物的每个锡原子具有两个Sn—R1键和一个Sn—OX键(其中OX是HOX的共轭酸,HOX是具有从0到6.8的pKa的Bronsted酸的群);和 由R2OCOOR2(其中R2代表线性或支链、饱和或不饱和碳氢基团、具有饱和或不饱和环烃取代基的碳氢基团,或Y—CH2—基团(其中Y代表烷基多聚烯基基团、芳香基团或环状饱和或不饱和烷基醚基团))表示的碳酸酯;和/或 由R2OH(其中R2与上述定义相同)表示的醇。
-
Direct transformation of trialkyl phosphates into organolithium compounds by a DTBB-catalysed lithiation
-
Preparation of novel substituted haloarene compounds申请人:Rutherford L. Jennifer公开号:US20060030714A1公开(公告)日:2006-02-09This invention relates to a new process for the preparation of novel substituted haloarene compounds of the formula I or IV: respectively, wherein R 1 , R 2 , R 3 , R 4 , R 5 , X, and Y are as defined herein, that comprises a novel and efficient selective mono-lithiation of a dihaloarene of the formula II or V: respectively, by an organo-lithium compound in the presence of a carbonyl reactant of the formula III: wherein R 1 and R 2 are as defined herein. In the process of the instant invention, the newly formed lithiated haloarene is sequentially quenched in situ by the carbonyl reactant to form said substituted haloarene. The process is suitable for batch or continuous flow systems. The substituted haloarenes produced by the process of the present invention are useful intermediates in the preparation of N-aryl or N-heteroaryl substituted pharmaceutically active compounds that include selective antagonists, inverse agonists and partial agonists of serotonin 1 (5-HT 1 ) receptors useful in treating or preventing depression, anxiety, obsessive compulsive disorder (OCD) and other disorders for which a 5-HT 1 agonist or antagonist is indicated.这项发明涉及一种新的制备式I或IV的新颖取代卤代芳烃化合物的过程:其中R1、R2、R3、R4、R5、X和Y如本文所定义,该过程包括一种新颖且高效的选择性对二卤代芳烃式II或V进行单锂化的步骤,通过在碳酰基试剂式III存在下使用有机锂化合物,其中R1和R2如本文所定义。在本发明的过程中,新形成的锂化卤代芳烃被碳酰基试剂原位顺序熄灭以形成所述的取代卤代芳烃。该过程适用于批处理或连续流系统。通过本发明的过程生产的取代卤代芳烃是制备N-芳基或N-杂环芳基取代的药用活性化合物的有用中间体,包括选择性5-羟色胺1(5-HT1)受体的拮抗剂、逆向激动剂和部分激动剂,用于治疗或预防抑郁症、焦虑症、强迫症(OCD)和其他需要5-HT1激动剂或拮抗剂的疾病。
表征谱图
-
氢谱1HNMR
-
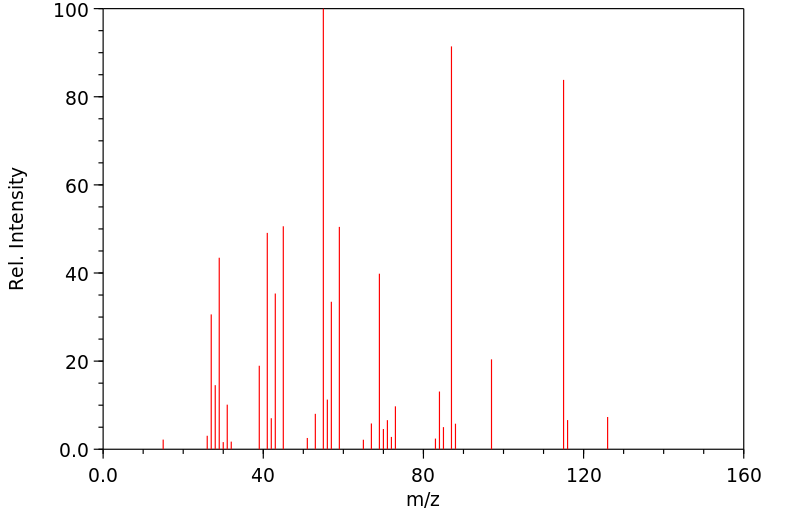
质谱MS
-
碳谱13CNMR
-
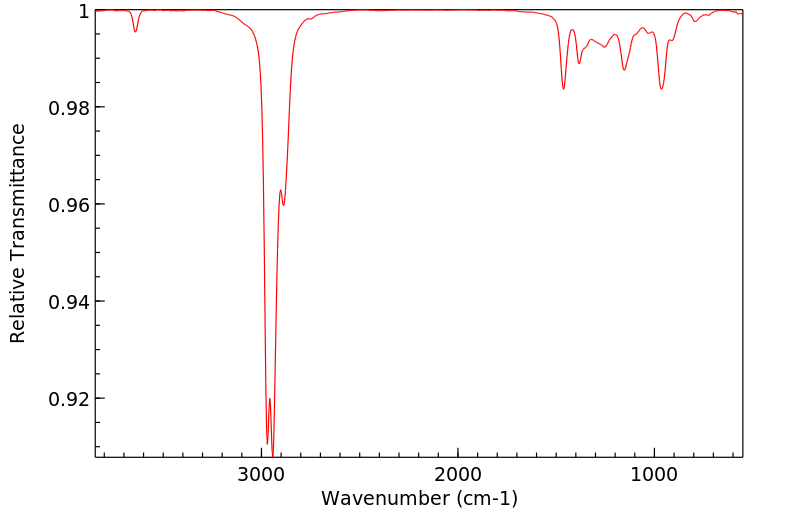
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷