5-十四碳炔 | 60212-34-2
中文名称
5-十四碳炔
中文别名
——
英文名称
5-tetradecyne
英文别名
tetradec-5-yne
CAS
60212-34-2
化学式
C14H26
mdl
——
分子量
194.36
InChiKey
WHRIRYDVFUEMDX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:2.51°C (estimate)
-
沸点:250.83°C (estimate)
-
密度:0.7976 (estimate)
-
保留指数:1421
计算性质
-
辛醇/水分配系数(LogP):6.6
-
重原子数:14
-
可旋转键数:8
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
反应信息
-
作为反应物:描述:5-十四碳炔 、 (氯二氟甲基)三甲基硅烷 在 四丁基氯化铵 作用下, 以 甲苯 为溶剂, 反应 4.0h, 以99%的产率得到1-butyl-3,3-difluoro-2-octylcycloprop-1-ene参考文献:名称:氯离子催化生成的二氟卡宾,可以有效地制备宝石级的二氟环丙烯和环丙烷。摘要:在较温和的中性条件下,由相对无毒且便宜的前驱体Me(3)SiCF(2)Cl(1)进行氯离子催化生成二氟卡宾,可通过[2]有效地制备宝石-二氟环丙烯和二氟环丙烷。 +1]分别与炔烃和烯烃的环加成反应。DOI:10.1039/c0cc04548a
-
作为产物:参考文献:名称:方便的还原烷基化的甲苯磺酰基甲基异氰酸酯:天然产物的合成应用1摘要:描述了一种方便和简单的方法,用于在液态氨中用锂将单和二烷基化的甲苯磺酰基甲基异氰化物还原为相应的烃。在Tricos-9Z-ene的合成中采用了这种方法。(7克)普通家蝇的性信息素,(-)-1S.5R,7S-exo-brevicomin(17),西松甲虫性信息素的对映体和(4S,5S)-5-羟基-4-癸内酯(L-因子,19),一种用于白细胞霉素生物合成的拟议的自动调节剂。DOI:10.1016/s0040-4020(01)86095-6
文献信息
-
Cross-Coupling of Nonactivated Alkyl Halides with Alkynyl Grignard Reagents: A Nickel Pincer Complex as the Catalyst作者:Oleg Vechorkin、Aurélien Godinat、Rosario Scopelliti、Xile HuDOI:10.1002/anie.201105964日期:2011.12.2In a pinch: The nickel pincer complex 1 catalyzes the cross‐coupling of the title compounds with remarkable substrate scope and functional group tolerance. A nickel/alkynyl species was isolated and shown to be catalytically competent. THF=tetrahydrofuran, O‐TMEDA=bis[2‐(N,N‐dimethylaminoethyl)] ether.
-
N-Heterocyclic carbene copper-catalyzed direct alkylation of terminal alkynes with non-activated alkyl triflates作者:Liqun Jin、Wangfang Hao、Jianeng Xu、Nan Sun、Baoxiang Hu、Zhenlu Shen、Weimin Mo、Xinquan HuDOI:10.1039/c7cc00891k日期:——(NHC)-Cu-catalyzed C(sp)-C(sp3) bond formation has been successfully achieved under mild conditions. Nonactivated alkyl triflates, which could be easily derived from alcohols, were utilized as C-O electrophiles. Mechanistic studies suggested that...
-
REACTION OF TRIALKYLBORANE WITH 1-ALKYNE AND LEAD(IV) ACETATE. A NEW REGIOSPECIFIC AND STEREOSPECIFIC ONE-POT SYNTHESIS OF ENOL ACETATES作者:Yuzuru Masuda、Masayuki Hoshi、Akira AraseDOI:10.1246/cl.1980.413日期:1980.4.5In the reaction of trialkylborane with 1-alkyne and lead(IV) acetate in hexane, one of the alkyl groups of trialkylborane migrated to the terminal carbon atom of the triple bond, giving regiospecifically an internal enol acetate and an internal alkyne as the main reaction products. The former compound had (Z)-configuration.
-
Synthesis of gem-Difluorinated Cyclopropanes and Cyclopropenes: Trifluoromethyltrimethylsilane as a Difluorocarbene Source作者:Fei Wang、Tao Luo、Jinbo Hu、Ying Wang、Hema S. Krishnan、Parag V. Jog、Somesh K. Ganesh、G. K. Surya Prakash、George A. OlahDOI:10.1002/anie.201101691日期:2011.7.25Highly versatile: The Ruppert–Prakash reagent (Me3SiCF3) can be an efficient source of difluorocarbene. By varying the nonmetallic initiator that is used (F− at lower temperatures and I− at higher temperatures), a range of structurally diverse alkenes and alkynes can be converted into the corresponding gem‐difluorinated cyclopropanes and cyclopropenes in good yields (see scheme).
-
368. Unsaturated fatty acids. Part I. The synthesis of erythrogenic (isanic) and other acetylenic acids作者:H. K. Black、B. C. L. WeedonDOI:10.1039/jr9530001785日期:——
表征谱图
-
氢谱1HNMR
-
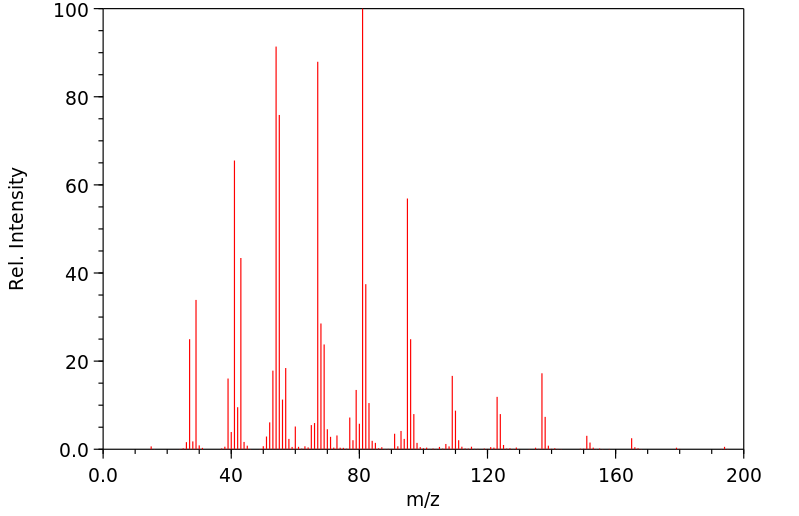
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-