(Z)-2-十一碳烯 | 821-96-5
中文名称
(Z)-2-十一碳烯
中文别名
——
英文名称
(Z)-2-undecene
英文别名
cis-2-undecene;(Z)-2-Undecen;cis-n-Undecen-(2);2-Undecene, (Z)-;(Z)-undec-2-ene
CAS
821-96-5
化学式
C11H22
mdl
——
分子量
154.296
InChiKey
JOHIXGUTSXXADV-HYXAFXHYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-66.5°C
-
沸点:196.15°C
-
密度:0.7801 (estimate)
-
LogP:6.082 (est)
-
保留指数:1111;1111;1118;1112;1102
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:11
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.82
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-十一烯 1-undecene 821-95-4 C11H22 154.296 十一碳-1,2-二烯 undeca-1,2-diene 56956-46-8 C11H20 152.28 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-2-undecene 693-61-8 C11H22 154.296
反应信息
-
作为反应物:描述:参考文献:名称:Fabre, Jean-Luc; Julia, Marc; Verpeaux, Jean-Noel, Bulletin de la Societe Chimique de France, 1985, # 5, p. 772 - 778摘要:DOI:
-
作为产物:描述:正十一胺 在 triphenylpyridine 作用下, 生成 (Z)-2-十一碳烯参考文献:名称:伯胺向烯烃的转化:霍夫曼消除的温和替代方案摘要:通过使用五环吡喃鎓盐(1),可以在温和的条件下将胺RR'CHCH 2 NH 2两步转化为RR'C CH 2。DOI:10.1039/c39810000096
文献信息
-
Hindered organoboron groups in organic chemistry. 25. The condensation of aliphatic aldehydes with dimesitylboryl stabilised carbanions to give alkenes.作者:Andrew Pelter、Keith Smith、Said M.A. ElgendyDOI:10.1016/s0040-4020(01)87983-7日期:1993.8In the presence of protic acids the condensation of aliphatic aldehydes with dimesitylboryl stabilised carbanions results in alkenes. In the presence of strong acids such as HCl or CF3SO3H, the products contain > 90% of E-alkenes in all cases tried. When acetic acid is used, then Z-alkenes may result predominantly, particularly in the cases of RsCHO and RtCHO. HX HCI, CF3SO3H gives E - alkenes in在质子酸的存在下,脂族醛与二聚三苯甲基稳定的碳负离子的缩合会生成烯烃。在强酸(例如HCl或CF 3 SO 3 H)的存在下,在所有尝试的情况下,产品均含有> 90%的E-烯烃。当使用乙酸时,则可能主要产生Z-烯烃,特别是在R s CHO和R t CHO的情况下。在所有情况下,HXHCl,CF 3 SO 3 H均会生成E-烯烃。HXCH 3 CO 2 H,使得主要ž -烯烃当Rř秒,R叔。
-
Organic synthesis with sulfones XXXVIII作者:Marc Julia、Hélène Lauron、Jean-Pierre Stacino、Jean-Noël Verpeaux、Yves Jeannin、Yves DromzeeDOI:10.1016/0040-4020(86)80011-4日期:1986.1The stereospecific hydrogenolysis of vinylic sulfones by sodium dithionite in a protic medium proceeds by addition of HSO2 to give an intermediate which could be isolated after alkylation in situ to a 1,2-bissulfone. The mechanism is therefore of the β-addition-elimination type. In the case of E-2-benzenesulfonyl-2-butene and ethyl iodide a single crystalline diastereoisomer was obtained and shown
-
Hydroindation of allenes and its application to radical cyclization作者:Naoki Hayashi、Yusuke Hirokawa、Ikuya Shibata、Makoto Yasuda、Akio BabaDOI:10.1039/b803314e日期:——Hydroindation of allenes and radical cyclization of 1,2,7-trienes (allenenes) were accomplished by HInCl2 with high regioselectivity to afford a variety of cyclic compounds. The resulting vinylic indiums could be used for successive coupling reactions in a one-pot procedure. The use of HInCl2 generated slowly in situ is extremely effective for the radical cyclization.
-
Hindered organoboron groups in organic synthesis. 14. stereoselective synthesis of alkenes by the boron-wittig reaction using aliphatic aldehydes作者:Andrew Pelter、Keith Smith、Said Elgendy、Martin RowlandsDOI:10.1016/s0040-4039(01)93821-3日期:1989.1In the presence of HX, carbanions Mes2BCHLiR1 react with aliphatic aldehydes to give alkenes. The stereochemistry of the product alkene depends upon the nature of HX.在HX存在下,碳负离子Mes 2 BCHLiR 1与脂族醛反应生成烯烃。产物烯烃的立体化学取决于HX的性质。
-
Iron Thiolate Complexes: Efficient Catalysts for Coupling Alkenyl Halides with Alkyl Grignard Reagents作者:Gérard Cahiez、Olivier Gager、Julien Buendia、Cindy PatinoteDOI:10.1002/chem.201200184日期:2012.5.7Ironing out the kinks: Efficient new catalytic systems based on iron thiolates are described for the iron‐catalyzed cross‐coupling of alkyl Grignard reagents with alkenyl halides (see scheme). The reaction is highly chemo‐ and stereoselective. With this new procedure, the use of N‐methylpyrrolidone as a co‐solvent is no longer required.
表征谱图
-
氢谱1HNMR
-
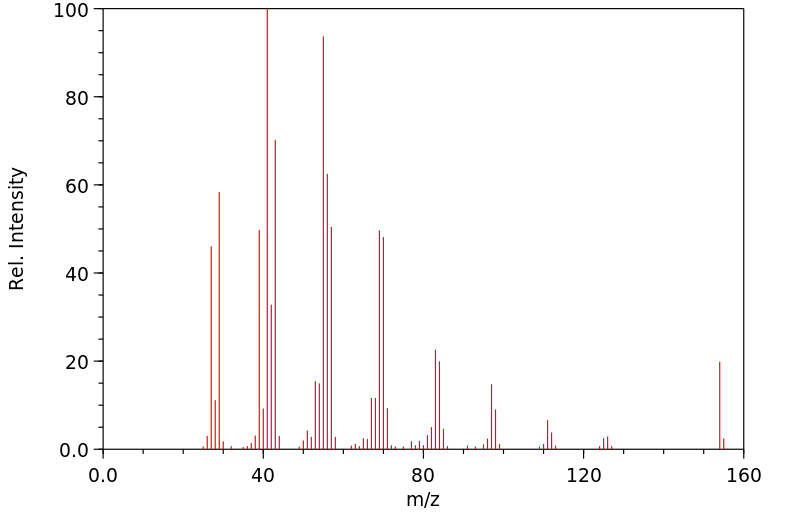
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-