hepta-1,5-diene | 1541-23-7
中文名称
——
中文别名
——
英文名称
hepta-1,5-diene
英文别名
1,5-heptadiene;1,5-Heptadien;Hepta-1,5-dien
CAS
1541-23-7
化学式
C7H12
mdl
——
分子量
96.1723
InChiKey
ZGXMNEKDFYUNDQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-105.67°C (estimate)
-
沸点:94.25°C
-
密度:0.72
-
闪点:-3 °C
-
LogP:3.295 (est)
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知的危险反应。请避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.43
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3
-
危险品运输编号:UN 3295
-
储存条件:请将贮藏器保持密封状态,并将其存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
1,5-庚二烯(顺反异构体混合物)
模块 1. 化学品
产品名称: 1,5-Heptadiene (cis- and trans- mixture)
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,5-庚二烯(顺反异构体混合物)
百分比: >97.0%(GC)
CAS编码: 1541-23-7
1,5-庚二烯(顺反异构体混合物)
模块 3. 成分/组成信息
分子式: C7H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
1,5-庚二烯(顺反异构体混合物)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: -3°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.72
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
1,5-庚二烯(顺反异构体混合物)
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1,5-Heptadiene (cis- and trans- mixture)
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
吸入性危害物质 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
若吞咽并进入呼吸道可能致命
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。切勿催吐。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
[储存] 存放于通风良好处。保持凉爽。
存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,5-庚二烯(顺反异构体混合物)
百分比: >97.0%(GC)
CAS编码: 1541-23-7
1,5-庚二烯(顺反异构体混合物)
模块 3. 成分/组成信息
分子式: C7H12
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(自携式呼吸器)。远离溢出物/泄露处并处在上风处。确保
紧急措施: 足够通风。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 半面罩或全面罩呼吸器,自携式呼吸器(SCBA),供气呼吸器等。依据当地和政府法
规,使用通过政府标准的呼吸器。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
1,5-庚二烯(顺反异构体混合物)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: -3°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.72
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
1,5-庚二烯(顺反异构体混合物)
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 3295
正式运输名称: 碳氢化合物, 液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
反应信息
-
作为反应物:描述:hepta-1,5-diene 在 dihydrogen hexachloroplatinate(IV) hexahydrate 、 三氯硅烷 、 碘 、 cesium fluoride 、 sodium t-butanolate 作用下, 以 异丙醇 、 N,N-二甲基甲酰胺 为溶剂, 反应 42.0h, 以40%的产率得到1,6-庚二烯参考文献:名称:通过链走加氢官能化和形式逆加氢官能化进行热力学烯烃异构化。摘要:我们报告了由还原反应和形成Si-F键驱动的内部烯烃对末端烯烃的反热力学异构化。该过程涉及内部烯烃的链走氢化硅烷化和随后的正式逆向氢化硅烷化。该方法取决于铂氢化硅烷化催化剂对金属烷基中间体的异构化的高活性,以及基于烷基硅烷向烯烃转化的无金属新方法。通过这种方法,将1,2-二取代和三取代的烯烃转化为末端烯烃。DOI:10.1021/acs.orglett.9b02695
-
作为产物:描述:参考文献:名称:Levy; Cope, Journal of the American Chemical Society, 1944, vol. 66, p. 1687摘要:DOI:
文献信息
-
Regioselective Carboindation of Simple Alkenes with Indium Tribromide and Ketene Silyl Acetals作者:Yoshihiro Nishimoto、Hiroki Ueda、Yoshihiro Inamoto、Makoto Yasuda、Akio BabaDOI:10.1021/ol1012108日期:2010.8.6The regioselective carboindation of simple alkenes with indium tribromide and ketene silyl acetals was accomplished. Various alkenes such as ethylene, 1-alkenes, and cyclic alkenes were applicable for this reaction system. The alkylindium product from the carboindation of cyclohexene revealed an anti addition mechanism.
-
Fe-catalyzed three-component dicarbofunctionalization of unactivated alkenes with alkyl halides and Grignard reagents作者:Lei Liu、Wes Lee、Cassandra R. Youshaw、Mingbin Yuan、Michael B. Geherty、Peter Y. Zavalij、Osvaldo GutierrezDOI:10.1039/d0sc02127j日期:——reported. The reaction operates under fast turnover frequency and tolerates a diverse range of sp2-hybridized nucleophiles (electron-rich and electron-deficient (hetero)aryl and alkenyl Grignard reagents), alkyl halides (tertiary alkyl iodides/bromides and perfluorinated bromides), and unactivated olefins bearing diverse functional groups including tethered alkenes, ethers, protected alcohols, aldehydes
-
Iridium-Catalyzed, Diastereoselective Dehydrogenative Silylation of Terminal Alkenes with (TMSO)<sub>2</sub>MeSiH作者:Chen Cheng、Eric M. Simmons、John F. HartwigDOI:10.1002/anie.201304084日期:2013.8.19achieved under mild conditions with low catalyst loading. The diastereoselectivity of the reaction can be controlled by choosing the appropriate ancillary ligand (see scheme; coe=cyclooctene). The silylation products undergo further transformation such as oxidation or cross‐coupling.
-
α-C—H Alkylation of Methyl Sulfides with Alkenes by a Scandium Catalyst作者:Yong Luo、Yuanhong Ma、Zhaomin HouDOI:10.1021/jacs.7b11245日期:2018.1.10addition of sulfides to alkenes is an atom-efficient route for the functionalization and modification of sulfide compounds through C-C bond formation, but this transformation is highly challenging. We report here the regioselective α-C(sp3)-H addition of a wide range of methyl sulfides to a variety of olefins and dienes by a half-sandwich scandium catalyst. This protocol provides a unique route for the synthesis
-
Asymmetric Hydrosilylation of 1-Alkenes Catalyzed by Palladium–MOP作者:Yasuhiro Uozumi、Kenji Kitayama、Tamio Hayashi、Kazunori Yanagi、Emiko FukuyoDOI:10.1246/bcsj.68.713日期:1995.3Asymmetric hydrosilylation of simple terminal alkenes (RCH=CH2) with trichlorosilane at 40 °C in the presence of 1 × 10−3 or 1 × 10−4 molar amounts of palladium catalyst prepared in situ from [PdCl(η3-C3H5)]2 and (S)-2-diphenylphosphino-2′-methoxy-1,1′-binaphthyl ((S)-MeO-MOP) proceeded with unusual regioselectivity and with high enantioselectivity to give high yields of 2-(trichlorosilyl)alkanes together在由 [PdCl(η3-C3H5)]2 原位制备的 1 × 10−3 或 1 × 10−4 摩尔量的钯催化剂存在下,简单末端烯烃 (RCH=CH2) 与三氯硅烷在 40 °C 的不对称氢化硅烷化(S)-2-二苯基膦基-2'-甲氧基-1,1'-联萘 ((S)-MeO-MOP) 以不寻常的区域选择性和高对映选择性进行,得到高产率的 2-(三氯甲硅烷基)烷烃少量的 1-(三氯甲硅烷基)烷烃。通过碳-硅键的氧化获得旋光醇,RCH(OH)CH3。相对于 1-甲硅烷基烷烃形成 2- 甲硅烷基烷烃的区域选择性和醇的对映体纯度如下:R = n-C4H9:89/11,94% ee (R)。R = n-C6H13:93/7 95% ee (R)。R = n-C10H21:94/6,95% ee (R)。R = Ph :81/19,97% ee (S)。R = Ph :80/20,92%
表征谱图
-
氢谱1HNMR
-
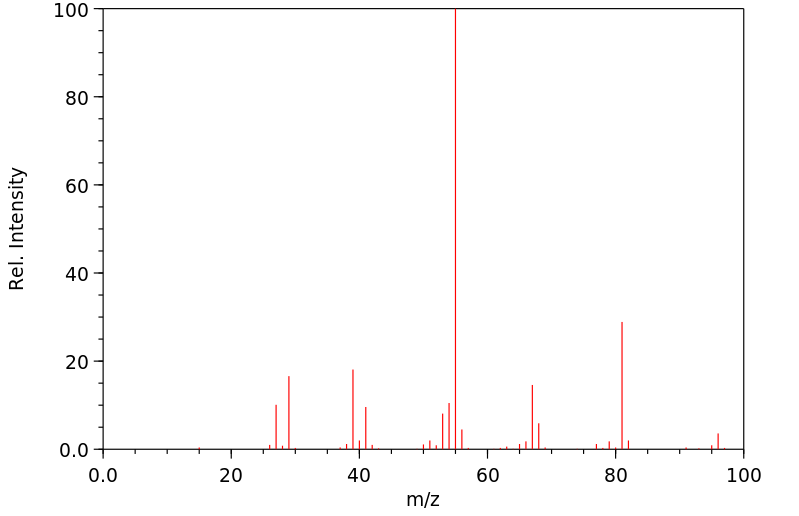
质谱MS
-
碳谱13CNMR
-
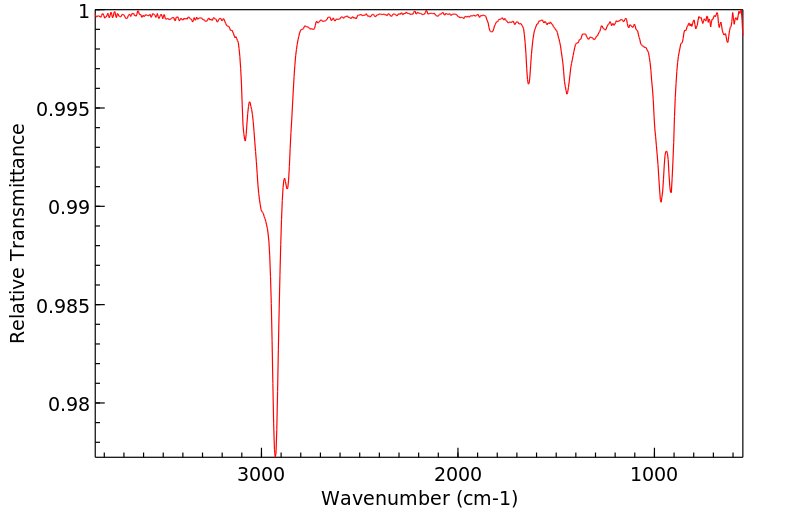
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-