1,3-二甲基环己烯 | 2808-76-6
中文名称
1,3-二甲基环己烯
中文别名
——
英文名称
1,3-dimethylcyclohexene
英文别名
1,3-Dimethyl-cyclohexen;1,3-Dimethyl-cyclohexen-(1);(+/-)-1,3-Dimethyl-cyclohex-1-en;1,3-Dimethyl-1-cyclohexene
CAS
2808-76-6
化学式
C8H14
mdl
MFCD00001555
分子量
110.199
InChiKey
XTCMQAVNRXZBRH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-81.99°C (estimate)
-
沸点:127.05°C
-
密度:0.7991
-
保留指数:825;836;813;825
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:8
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Iodo-1,3-dimethylcyclohexene 158362-89-1 C8H13I 236.096
反应信息
-
作为反应物:描述:参考文献:名称:Aromatization of cyclohexenes and cyclohexadienes with selenium dioxide-trimethylsilyl polyphosphate摘要:Selenium dioxide is depolymerized and activated by trimethylsilyl polyphosphate in carbon tetrachloride. The reagent effectively aromatizes substituted cyclohexenes and cyclohexadienes under mild reaction conditions.DOI:10.1016/s0040-4039(00)60974-7
-
作为产物:描述:参考文献:名称:α-生育酚的合成研究。三、Phytone的合成摘要:Phytone (XI) 是制备 α-生育酚的重要中间体,通过香叶基三苯基卤化鏻 (VII) 与 2-甲基-6-氧代庚醛 (VIII) 的醛基之间的选择性 Wittig 反应合成,然后氢化产品。VIII 是由 1,3-二甲基-1-环己烯 (V) 通过臭氧分解和还原分解制备的。通过 2,6-二甲基环己-1-醇 (II) 的脱水无法获得纯态的 V,可能是因为中间体碳正离子的异构化。发现 II (III) 的乙酸酯的热解得到纯 V。 III 的难以热解的立体异构体是顺式,顺式-2,6-二甲基环己基-1-乙酸酯 (VI) 与顺式消除机制一致.DOI:10.1246/bcsj.41.1232
文献信息
-
Stereochemistry of Hydrodenitrogenation: The Mechanism of Elimination of the Amino Group from Cyclohexylamines over Sulfided Ni–Mo/γ-Al2O3 Catalysts作者:Fabio Rota、Vidyadhar S Ranade、Roel PrinsDOI:10.1006/jcat.2001.3218日期:2001.6cyclohexylamines occurs primarily by means of a β elimination mechanism. Elimination of a β hydrogen atom attached to a tertiary carbon atom is faster than that of a β hydrogen atom attached to a secondary carbon atom. The rate of elimination also depended on the stereochemical configuration of the amino group with respect to the β hydrogen atoms. Elimination was more rapid when the configuration of the环己基胺和2-甲基环己胺和2,6- dimethylcyclo己胺的非对映体的加氢在超过一个硫化镍-钼/γ-Al系200〜350℃和50巴压力进行了研究2 ö 3催化剂。从环己胺中去除氨基主要是通过β消除机理进行的。与叔碳原子连接的β氢原子的消除比与仲碳原子连接的β氢原子的消除快。消除速率还取决于氨基相对于β氢原子的立体化学构型。当氨基的构型相对于β氢原子(在椅子构象上)反平面时,消除的速度要比消除β原子时的消除更快。syn平顶面(船形)。这与E2消除机制一致。根据氨基和β氢原子之间的立体化学关系,β氢原子的数量和类型以及获得所需椅子/船体所需的能量,对取代环己胺的所有非对映异构体的相对消除率进行了合理化处理。构象。与先前提出的从醇中抗除H 2 O的提议相反,已经表明,如果SH或OH基团从催化剂表面突出,则在异相催化剂表面上可能存在抗消除机理。
-
Hydrogenation of arenes and N-heteroaromatic compounds over ruthenium nanoparticles on poly(4-vinylpyridine): a versatile catalyst operating by a substrate-dependent dual site mechanism作者:Minfeng Fang、Nataliya Machalaba、Roberto A. Sánchez-DelgadoDOI:10.1039/c1dt10801h日期:——A nanostructured catalyst composed of Ru nanoparticles immobilized on poly(4-vinylpyridine) (PVPy) has been synthesized by NaBH4 reduction of RuCl3·3H2O in the presence of the polymer in methanol at room temperature. TEM measurements show well-dispersed Ru nanoparticles with an average diameter of 3.1 nm. Both powder XRD patterns and XPS data indicate that the Ru particles are predominantly in the zerovalent state. The new catalyst is efficient for the hydrogenation of a wide variety of aromatic hydrocarbons and N-heteroaromatic compounds representative of components of petroleum-derived fuels. The experimental data indicate the existence of two distinct active sites in the nanostructure that lead to two parallel hydrogenation pathways, one for simple aromatics involving conventional homolytic hydrogen splitting on Ru and a second one for N-heteroaromatics taking place via a novel heterolytic hydrogen activation on the catalyst surface, assisted by the basic pyridine groups of the support.
-
Synthesis of olefins from sterically hindered alcohols by pyrolysis of thiocarbonate O-esters作者:H. Gerlach、Tran Thi Houng、W. MüllerDOI:10.1039/c39720001215日期:——Sterically hindered alcohols e.g.(I; R = H) were acylated with O-4-methylphenyl chlorothioformate in high yield and the resulting thiocarbonates were converted into olefins by pyrolysis.
-
Diastereoselective Epoxidation of Cyclohexene Derivatives by Dioxiranes Generated in Situ. Importance of Steric and Field Effects作者:Dan Yang、Guan-Sheng Jiao、Yiu-Chung Yip、Man-Kin WongDOI:10.1021/jo9821978日期:1999.3.1In this paper, diastereoselective epoxidation of substituted cyclohexenes (substrates 1-7) by dioxiranes generated in situ from ketones and Oxone was systematically investigated. The results revealed that the diastereoselectivity was determined by the steric and field effects of both dioxiranes and substrates, and high diastereoselectivity can be achieved by tuning the ketone structure. Among the ketones本文系统地研究了由酮和Oxone原位生成的二恶环酮对取代的环己烯(底物1-7)进行非对映选择性环氧化。结果表明,非对映选择性是由二恶英酮和底物的空间和场效应决定的,通过调节酮的结构可以实现高非对映选择性。在测试的酮中,12和19的非对映选择性最佳。
-
A Novel Epoxidation Reaction of Olefins Using a Combination of Chloramine-M, Benzaldehyde, and Benzyltriethylammonium Chloride作者:Dan Yang、Chi Zhang、Xue-Chao WangDOI:10.1021/ja993472q日期:2000.5.1products. Good to excellent diastereoselectivities were obtained for epoxidation of two substituted cyclohexenes. Chloramine-T was found to give a slower reaction than Chloramine-M. cis-N-Sulfonyloxaziridine D is proposed to be the epoxidizing agent in this novel epoxidation reaction on the basis of the mechanistic studies.
表征谱图
-
氢谱1HNMR
-
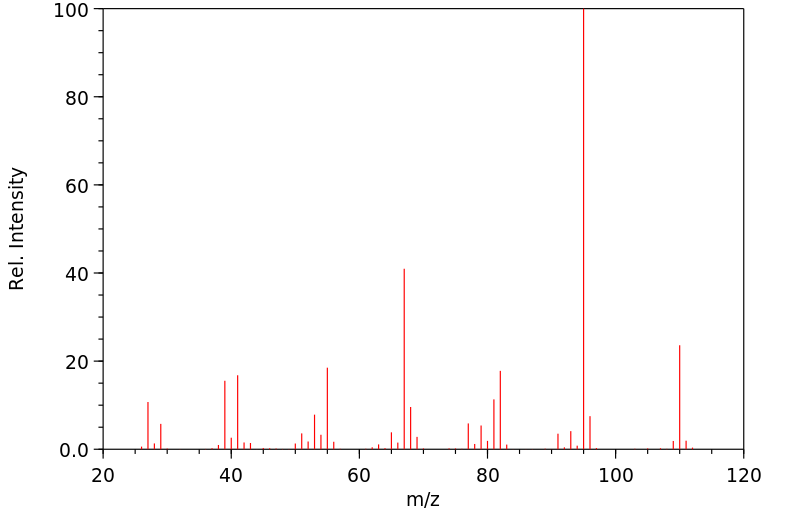
质谱MS
-
碳谱13CNMR
-
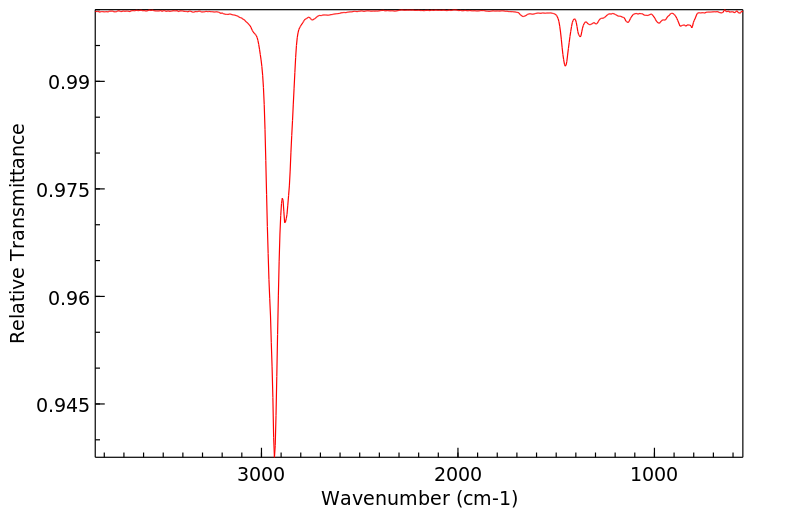
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-