2-二甲氨基甲基环己酮 | 15409-60-6
中文名称
2-二甲氨基甲基环己酮
中文别名
2-[(二甲基氨基)甲基]-环己烷;2-二甲氨基甲基-1-环己酮;2-(N,N-二甲基氨基甲基)环己酮
英文名称
2-[(dimethylamino)methyl]cyclohexanone
英文别名
2-((dimethylamino)methyl)cyclohexan-1-one;2-((Dimethylamino)methyl)cyclohexanone;2-[(dimethylamino)methyl]cyclohexan-1-one
CAS
15409-60-6
化学式
C9H17NO
mdl
MFCD00019457
分子量
155.24
InChiKey
QDHLEFBSGUGHCL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:148-150 °C
-
沸点:222℃
-
密度:0.944
-
闪点:70℃
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:20.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:2-8°C
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基环己基甲基胺 N,N-dimethylcyclohexylmethylamine 16607-80-0 C9H19N 141.257
反应信息
-
作为反应物:描述:2-二甲氨基甲基环己酮 在 L-Selectride 作用下, 以 四氢呋喃 为溶剂, 反应 3.0h, 以56%的产率得到(+/-)-cis-1-(Dimethylamino-methyl)-cyclohexanol-(2)参考文献:名称:Gregan; kettmann; Novomesky, Il Farmaco, 1995, vol. 50, # 12, p. 829 - 839摘要:DOI:
-
作为产物:描述:2-((二甲氨基)亚甲基)环己酮 在 lithium aluminium tetrahydride 作用下, 以 乙醚 为溶剂, 以98%的产率得到2-二甲氨基甲基环己酮参考文献:名称:经由烯胺酮从酮和酯合成曼尼希碱。摘要:描述了一系列活化的亚甲基化合物与酰胺缩醛的反应以形成高产率的烯胺酮。还涵盖了通过用氢化锂铝还原而进一步转化为曼尼希碱的方法。DOI:10.1016/s0040-4039(00)84586-4
-
作为试剂:描述:间碘苯腈 在 异丙基氯化镁 、 2-二甲氨基甲基环己酮 作用下, 以 四氢呋喃 为溶剂, 反应 1.5h, 以73%的产率得到3-[[(2-dimethylamino)methyl]-1-hydroxycyclohexyl]-benzonitrile参考文献:名称:Carboxamido opioid compounds摘要:这项发明涉及羧酰胺类阿片类化合物,作为治疗或调节中枢神经系统疾病的药物,并提供了治疗或调节中枢神经系统疾病的方法。公开号:US20050256203A1
文献信息
-
Opioid Detection申请人:Randox Laboratories Limited公开号:US20170176476A1公开(公告)日:2017-06-22An immunoassay method is described which detects O-desmethyltramadol only. This enables an assay of high sensitivity and specificity avoiding false positive results. The unique antibodies incorporated in the immunoassay method can be combined with antibodies which detect mitragynine to provide an assay which increases the possibility of detecting the commonly found drug combination of O-desmethyltramadol and mitragynine.
-
A Novel Method for Biomimetic Synthesis of Mannich Bases作者:Yuan Guo、Jing An、Zhenhuan Lu、Mengjiao PengDOI:10.1002/cjoc.201100628日期:2012.7reaction has become an important tool for the synthesis of new compounds. Mannich bases can be either directly employed or used as intermediates. In this work, the one‐carbon unit transfer reaction of tetrahydrofolate coenzyme was initiated. 1,3‐Dimethylimidazolidine as a new tetrahydrofolate coenzyme model at formaldehyde oxidation level was used to react with ketone having active hydrogen atoms and amine
-
Reaction of Grignard reagents with carbonyl compounds under continuous flow conditions作者:E. Riva、S. Gagliardi、M. Martinelli、D. Passarella、D. Vigo、A. RencurosiDOI:10.1016/j.tet.2010.02.078日期:2010.4This contribution details how a continuous flow reactor was used to react carbonyl compounds with Grignard reagents at room temperature in an efficient and safe manner. Flow rate, residence time and temperature were optimized for the preparation of a small collection of secondary and tertiary alcohols. Excellent yields and general applicability were observed using the set-up protocol. The procedure
-
A Convenient Regioselective Synthesis of Mannich Bases
-
Palladium-Promoted Transformation of β-Amino Ketones to Enaminones作者:Shun-Ichi Murahashi、Yo Mitsue、Tatsuo TsumiyamaDOI:10.1246/bcsj.60.3285日期:1987.9The reaction of β-amino ketones with bis(acetonitrile)dichloropalladium(II) in the presence of triethylamine gives the corresponding enaminones regioselectively. The cyclic β-amino ketones can be converted into the corresponding exocyclic enaminones. The enaminones thus obtained are versatile synthetic intermediates. The reaction of (E)-enaminones with organocuprates gave the corresponding (E)-α,β-unsaturated ketones.
表征谱图
-
氢谱1HNMR
-
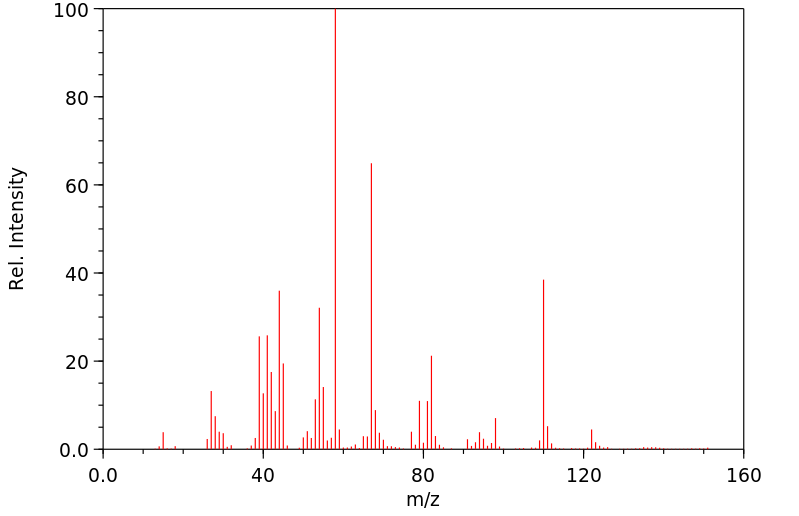
质谱MS
-
碳谱13CNMR
-
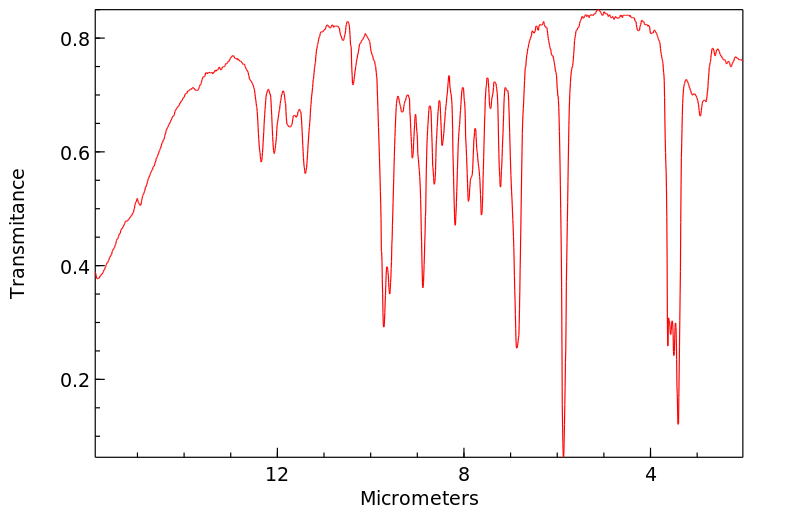
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙腈)二氯镍(II)
(R)-(-)-α-甲基组胺二氢溴化物
(N-(2-甲基丙-2-烯-1-基)乙烷-1,2-二胺)
(4-(苄氧基)-2-(哌啶-1-基)吡啶咪丁-5-基)硼酸
(11-巯基十一烷基)-,,-三甲基溴化铵
鼠立死
鹿花菌素
鲸蜡醇硫酸酯DEA盐
鲸蜡硬脂基二甲基氯化铵
鲸蜡基胺氢氟酸盐
鲸蜡基二甲胺盐酸盐
高苯丙氨醇
高箱鲀毒素
高氯酸5-(二甲氨基)-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-2-甲基吡啶正离子
高氯酸2-氯-1-({(E)-[4-(二甲氨基)苯基]甲亚基}氨基)-6-甲基吡啶正离子
高氯酸2-(丙烯酰基氧基)-N,N,N-三甲基乙铵
马诺地尔
马来酸氢十八烷酯
马来酸噻吗洛尔EP杂质C
马来酸噻吗洛尔
马来酸倍他司汀
顺式环己烷-1,3-二胺盐酸盐
顺式氯化锆二乙腈
顺式吡咯烷-3,4-二醇盐酸盐
顺式双(3-甲氧基丙腈)二氯铂(II)
顺式3,4-二氟吡咯烷盐酸盐
顺式1-甲基环丙烷1,2-二腈
顺式-二氯-反式-二乙酸-氨-环己胺合铂
顺式-二抗坏血酸(外消旋-1,2-二氨基环己烷)铂(II)水合物
顺式-N,2-二甲基环己胺
顺式-4-甲氧基-环己胺盐酸盐
顺式-4-环己烯-1.2-二胺
顺式-4-氨基-2,2,2-三氟乙酸环己酯
顺式-3-氨基环丁烷甲腈盐酸盐
顺式-2-羟基甲基-1-甲基-1-环己胺
顺式-2-甲基环己胺
顺式-2-(苯基氨基)环己醇
顺式-2-(苯基氨基)环己醇
顺式-2-(氨基甲基)-1-苯基环丙烷羧酸盐酸盐
顺式-1,3-二氨基环戊烷
顺式-1,2-环戊烷二胺二盐酸盐
顺式-1,2-环戊烷二胺
顺式-1,2-环丁腈
顺式-1,2-双氨甲基环己烷
顺式--N,N'-二甲基-1,2-环己二胺
顺式-(R,S)-1,2-二氨基环己烷铂硫酸盐
顺式-(2-氨基-环戊基)-甲醇
顺-2-戊烯腈
顺-1,3-环己烷二胺
顺-1,3-双(氨甲基)环己烷