5-碘-2-甲氧基吡啶 | 13472-61-2
中文名称
5-碘-2-甲氧基吡啶
中文别名
2-甲氧基-5-碘吡啶;6-甲氧基-3-碘吡啶;5-碘-2-甲氧吡啶
英文名称
5-iodo-2-methoxypyridine
英文别名
2-methoxy-5-iodopyridine
CAS
13472-61-2
化学式
C6H6INO
mdl
MFCD07781180
分子量
235.024
InChiKey
NTXRNCUPGYOZCN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:237℃
-
密度:1.825
-
闪点:97℃
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.166
-
拓扑面积:22.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2933399090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
5-Iodo-2-methoxypyridine
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
5-Iodo-2-methoxypyridine
Ingredient name:
CAS number: 13472-61-2
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H6INO
Molecular weight: 235
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
5-Iodo-2-methoxypyridine
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
5-Iodo-2-methoxypyridine
Ingredient name:
CAS number: 13472-61-2
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C6H6INO
Molecular weight: 235
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen Iodide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:叔丁基亚磺酰胺辅助剂的镁基非对映选择性制备吡啶基1,3-氨基醇摘要:摘要 通过使用两步法制备1,3-氨基醇,并讨论了非对映选择性的有趣逆转。 通过使用两步法制备1,3-氨基醇,并讨论了非对映选择性的有趣逆转。DOI:10.1055/s-0034-1378854
-
作为产物:描述:参考文献:名称:二丁基(异丙基)镁酸锂(1-)和氯化锂通过卤素-镁交换进行非低温合成功能化的2-甲氧基吡啶摘要:使用二丁基(异丙基)镁酸锂(1-)和相应的溴或碘类似物制备在3-,5-或6-位官能化的2-甲氧基吡啶和在3-位官能化的2,6-二甲氧基吡啶。非低温条件下的氯化锂。根据在两种镁酸盐之间的选择以及反应介质中氯化锂的存在与否,对程序进行了优化。 吡啶-镁-锂-有机金属试剂DOI:10.1055/s-0031-1289687
-
作为试剂:描述:1-氯-1-苯基丙烷 在 乙二醇二甲醚溴化镍 、 5-碘-2-甲氧基吡啶 、 三甲基氯硅烷 、 (4S,4'S)-4,4'-di(heptan-4-yl)-4,4',5,5'-tetrahydro-2,2'-bioxazole 、 锌 作用下, 以 1,4-二氧六环 为溶剂, 反应 18.0h, 以26%的产率得到3,4-diphenyl-hexane参考文献:名称:镍催化不对称还原交叉偶联获得 1,1-二芳基烷烃摘要:已经开发了(杂)芳基碘化物和苄基氯化物的不对称 Ni 催化还原交叉偶联,以制备对映体富集的 1,1-二芳基烷烃。作为这些研究的一部分,开发了一种新的手性生物恶唑啉配体 4-庚基-BiOX (L1),以便以合成有用的产率和对映选择性获得产品。该反应耐受多种杂环偶联物,包括吡啶、嘧啶、吲哚和哌啶。DOI:10.1021/jacs.7b01705
文献信息
-
Synthesis of Pyridylsulfonium Salts and Their Application in the Formation of Functionalized Bipyridines作者:Vincent K. Duong、Alexandra M. Horan、Eoghan M. McGarrigleDOI:10.1021/acs.orglett.0c03048日期:2020.11.6arylation of pyridylsulfides with good functional group tolerance was developed. To demonstrate synthetic utility, the resulting pyridylsulfonium salts were used in a scalable transition-metal-free coupling protocol, yielding functionalized bipyridines with extensive functional group tolerance. This modular methodology permits selective introduction of functional groups from commercially available pyridyl
-
Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins through Nickel-Catalyzed Cross-Electrophile Coupling作者:Hai-Yong Tu、Fang Wang、Liping Huo、Yuanbo Li、Shengqing Zhu、Xian Zhao、Huan Li、Feng-Ling Qing、Lingling ChuDOI:10.1021/jacs.0c03708日期:2020.5.27A nickel-catalyzed, enantioselective, three-component fluoroalkylarylation of unactivated alkenes with aryl halides and perfluoroalkyl iodides has been described. This cross-electrophile coupling protocol utilizes a chiral nickel/BiOx system as well as a pendant chelating group to facilitate the challenging three-component, asymmetric difunctionalization of unactivated alkenes, providing direct access
-
COMPOUNDS AND METHODS FOR TREATING OXALATE-RELATED DISEASES申请人:OxaluRx, Inc.公开号:US20210052586A1公开(公告)日:2021-02-25Disclosed herein are compounds and compositions for modulating glycolate oxidase, useful for treating oxalate-related diseases, such as hyperoxaluria, where modulating glycolate oxidase is expected to be therapeutic to a patent in need thereof. Methods of modulating glycolate oxidase activity in a human or animal subject are also provided.
-
[EN] BENZOTHIADIAZINE COMPOUNDS<br/>[FR] COMPOSÉS BENZOTHIADIAZINE申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2017098421A1公开(公告)日:2017-06-15The invention is directed to substituted benzothiadiazine derivatives. Specifically, the invention is directed to compounds according to Formula (I):wherein R, R1, R2, R3, R4 and R5 are as defined herein. The compounds of the invention are inhibitors of CD73 and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases associated with CD73 inhibition, such as AIDS, autoimmune diseases, infections, atherosclerosis, and ischemia-reperfusion injury. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting CD73 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
-
[EN] ETHYNE DERIVATIVES, PHARMACEUTICAL COMPOSITIONS AND USES THEREOF<br/>[FR] DÉRIVÉS D'ÉTHYNE, COMPOSITIONS PHARMACEUTIQUES ET LEURS UTILISATIONS申请人:BOEHRINGER INGELHEIM INT公开号:WO2012032014A1公开(公告)日:2012-03-15invention relates to new compounds of the formula I to their use as medicaments, to methods for their therapeutic use and to pharmaceutical compositions containing them.这项发明涉及公式I的新化合物,涉及它们作为药物的用途,涉及它们的治疗用途的方法,以及含有它们的药物组合物。
表征谱图
-
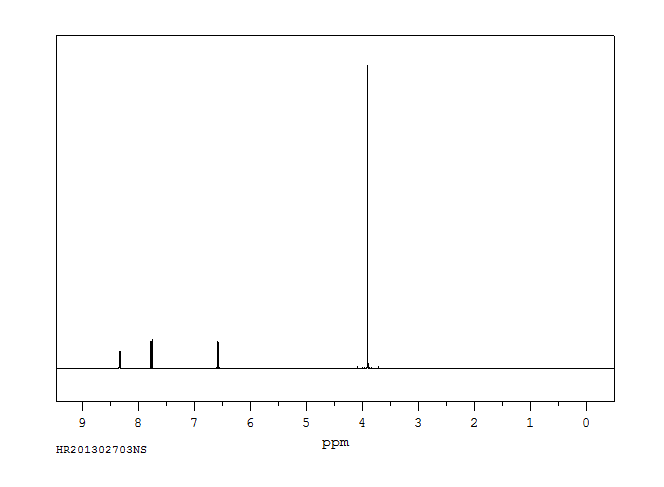
氢谱1HNMR
-
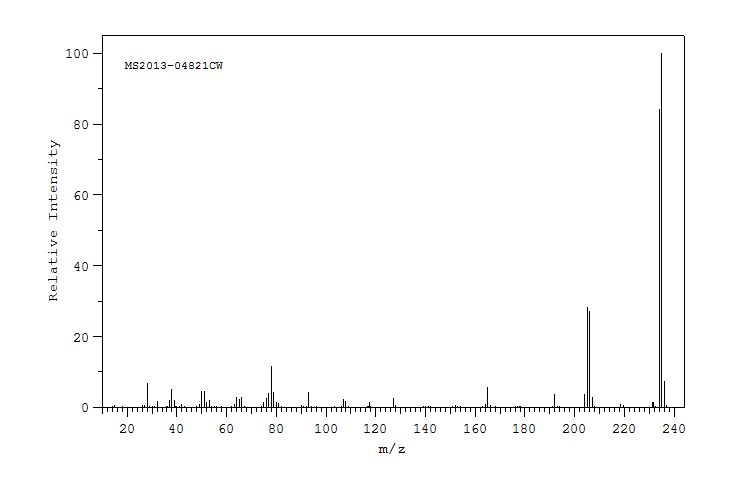
质谱MS
-
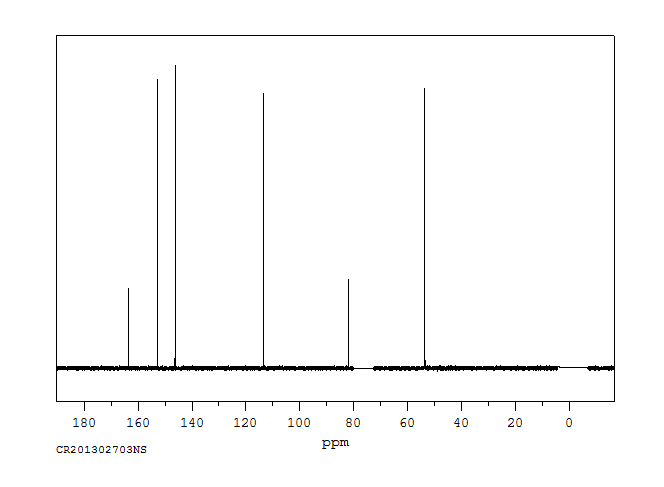
碳谱13CNMR
-
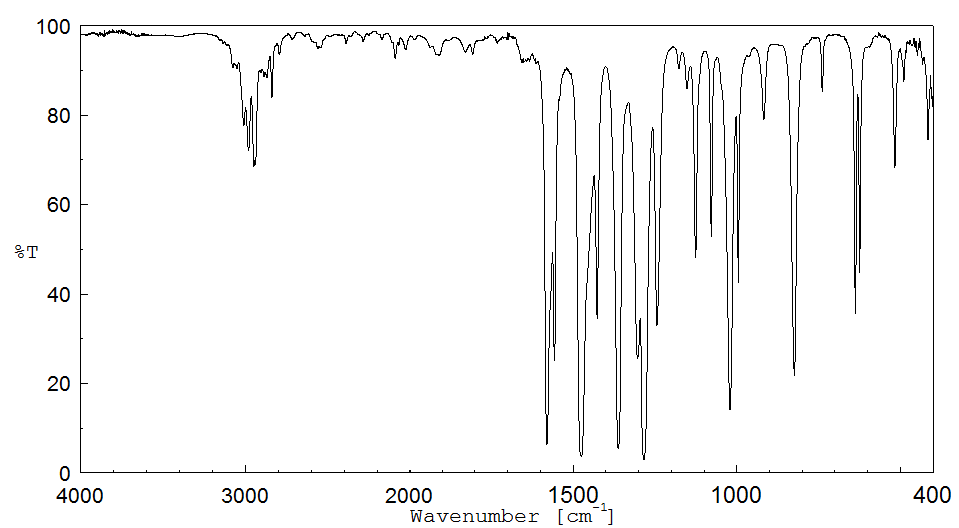
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷