DL-aspartic acid dimethyl ester hydrochloride | 32213-95-9
中文名称
——
中文别名
——
英文名称
DL-aspartic acid dimethyl ester hydrochloride
英文别名
dimethyl aspartate hydrochloride;aspartic acid dimethyl ester hydrochloride;(1,4-Dimethoxy-1,4-dioxobutan-2-yl)azanium;chloride
CAS
32213-95-9
化学式
C6H11NO4*ClH
mdl
——
分子量
197.619
InChiKey
PNLXWGDXZOYUKB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115-117 °C(lit.)
-
比旋光度:15 º (c=1, MeOH)
-
溶解度:DMSO(少许)、甲醇(少许)、水(少许)
计算性质
-
辛醇/水分配系数(LogP):-0.53
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:78.6
-
氢给体数:2
-
氢受体数:5
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2922499990
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
SDS
制备方法与用途
应用
L-天冬氨酸的二甲酯盐酸盐是白色晶体,在生物合成化学、药物化学、食品加工和光学活性材料等领域中作为重要原料。
制备
在磁力搅拌下,将氯化亚砜缓慢滴加到无水甲醇中,并控制温度在-10~-5 ℃之间。滴加完毕后,保持-10 ℃持续搅拌并反应一段时间(t A),以生成氯化亚硫酸甲酯。随后,分批加入相应的L-天冬氨酸,在恒定温度T A下继续搅拌反应一段时间(t B)。
接着进行减压蒸馏,加入甲醇,再重复几次减压蒸馏步骤,尽量除去未反应的氯化亚砜以及生成的氯化氢,使产物以固体形式析出。使用溶剂重结晶时,需注意甲醇的比例:比例过高,则产物较难析出且产率较低;比例过低,则产物不易溶解,需要更多的溶剂量。当甲醇和乙酸乙酯的体积比为1∶20时,重结晶效率最高,产率为95%。
反应信息
-
作为反应物:描述:DL-aspartic acid dimethyl ester hydrochloride 在 溶剂黄146 、 三乙胺 作用下, 以 氯仿 为溶剂, 反应 20.0h, 生成 Dimethyl 2-[2-(2,2,2-trichloroacetyl)pyrrol-1-yl]butanedioate参考文献:名称:Novel, Highly Potent Aldose Reductase Inhibitors: (R)-(−)-2-(4-Bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazine- 4-spiro-3‘-pyrrolidine-1,2‘,3,5‘-tetrone (AS-3201) and Its Congeners摘要:A series of novel tetrahydropyrrolo[1,2-a]pyrazine derivatives were synthesized and evaluated as aldose reductase inhibitors (ARIs) on the basis of their abilities to inhibit porcine lens aldose reductase (AR) in vitro and to inhibit sorbitol accumulation in the sciatic nerve of streptozotocin-induced diabetic rats in vivo. Of these compounds, spirosuccinimide-fused tetrahydropyrrolo[1,2-a]pyrazine-1,3-dione derivatives showed significantly potent AR inhibitory activity. In the in vivo activity of these derivatives, 2-(4-bromo-2-fluorobenzyl)-1,2,3,4-tetrahydropyrrolo- [1,2-a][1,2a]pyrazine-4-spiro-3'-pyrrolidine-1,2',3,5'-tetrone (23t) (SX-3030) showed the best oral activity. The enantiomers of 23t were synthesized, and the biological activities were evaluated. It was found that AR inhibitory activity resides in the (-)-enantiomer 43 (AS-3201), which was 10 times more potent in inhibition of the AR (IC50 = 1.5 x 10(-8) M) and 500 times more potent in the in vivo activity (ED50 = 0.18 mg/kg/day for 5 days) than the corresponding (+)enantiomer 44 (SX-3202): From these results, AS-3201 was selected as the candidate for clinical development. The absolute configuration of AS-3201 was also established to be (R)-form by single-crystal X-ray analysis. In this article we report the preparation and structure-activity relationship (SAR) of tetrahydropyrrolopyrazine derivatives including a novel ARI, AS-3201.DOI:10.1021/jm9802968
-
作为产物:参考文献:名称:一种方便合成的氨基酸甲酯。摘要:在三甲基氯硅烷存在下,通过氨基酸与甲醇的室温反应,制备了一系列氨基酸甲酯盐酸盐,收率良好至极好。该方法不仅与天然氨基酸相容,而且与其他芳香族和脂肪族氨基酸相容。DOI:10.3390/molecules13051111
文献信息
-
SUBSTITUTED BRIDGED UREA ANALOGS AS SIRTUIN MODULATORS申请人:GLAXOSMITHKLINE LLC公开号:US20150152108A1公开(公告)日:2015-06-04The present invention relates to novel substituted bridged urea compounds, corresponding related analogs, pharmaceutical compositions and methods of use thereof. Sirtuin-modulating compounds of the present invention may be used for increasing the lifespan of a cell, and treating and/or preventing a wide variety of diseases and disorders, which include, but are not limited to, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing as well as diseases or disorders that would benefit from increased mitochondrial activity. The present invention also related to compositions comprising a sirtuin-modulating compound in combination with another therapeutic agent.本发明涉及新型取代桥式脲化合物,相应的相关类似物,药物组合物以及其使用方法。本发明的抑制素调节化合物可用于延长细胞寿命,并治疗和/或预防各种疾病和疾病,包括但不限于与衰老或压力、糖尿病、肥胖、神经退行性疾病、心血管疾病、血液凝块疾病、炎症、癌症和/或潮红有关的疾病或疾病,以及那些会受益于增加线粒体活性的疾病或疾病。本发明还涉及包含抑制素调节化合物与另一治疗剂组合的组合物。
-
[EN] SUBSTITUTED BRIDGED UREA ANALOGS AS SIRTUIN MODULATORS<br/>[FR] ANALOGUES D'URÉE PONTÉS SUBSTITUÉS EN TANT QUE MODULATEURS DE SIRTUINE申请人:GLAXOSMITHKLINE IP NO 2 LTD公开号:WO2016079709A1公开(公告)日:2016-05-26The present invention relates to novel substituted bridged urea analog compounds of Formula (I) or pharmaceutically acceptable salts thereof, corresponding pharmaceutical compositions, processes for making and use of such compounds, alone or in combination with other therapeutic agents, as Sirtuin Modulators useful for increasing lifespan of a cell, and for use in treating and/or preventing a wide variety of diseases and disorders, which include, but are not limited to, for example, diseases or disorders related to aging or stress, diabetes, obesity, neurodegenerative diseases, cardiovascular disease, blood clotting disorders, inflammation, cancer, and/or flushing as well as diseases or disorders that would benefit from increased mitochondrial activity.
-
COMPOSITIONS AND METHODS FOR DETECTING NERVE AGENTS申请人:Corcoran Robert C.公开号:US20100130757A1公开(公告)日:2010-05-27The present invention provides methods and compositions for detecting, identifying and measuring the abundance of chemical nerve agents. Methods and compositions of the present invention are capable of providing selective detection of phosphorous based nerve agents, such as nerve agents that are esters of methyl phosphonic acid derivatives incorporating a moderately good leaving group at the phosphorus. Selectivity in the present invention is provided by a sensor composition having an alpha (α) effect nucleophile group that undergoes specific nucleophilic substitution and rearrangement reactions with phosphorus based nerve agents having a tetrahederal phosphorous bound to oxygen. The present invention includes embodiments employing a sensor composition further comprising a reporter group covalently linked to the alpha effect nucleophile group allowing rapid optical readout of nerve agent detection events, including direct visual readout and optical readout via spectroscopic analysis.
-
Microwave-Assisted Ruthenium-Catalysed <i>ortho</i> -C−H Functionalization of <i>N</i> -Benzoyl <i>α</i> -Amino Ester Derivatives作者:Nandini Sharma、Vijay Bahadur、Upendra K. Sharma、Debasmita Saha、Zhenghua Li、Yogesh Kumar、Jona Colaers、Brajendra K Singh、Erik V. Van der EyckenDOI:10.1002/adsc.201800458日期:2018.8.17A microwave‐assisted highly efficient intermolecular C−H functionalization sequence has been developed to access substituted isoquinolones using α‐amino acid esters as a directing group. This methodology enables a wide range of N‐benzoyl α‐amino ester derivatives to react via a Ru‐catalysed C−H bond activation sequence, to form isoquinolones with moderate to excellent yields. As an additional advantage
-
Reactivity and mechanism of α-nucleophile scaffolds as catalytic organophosphate scavengers作者:Pamela T. Wong、Somnath Bhattacharjee、Jayme Cannon、Shengzhuang Tang、Kelly Yang、Sierra Bowden、Victoria Varnau、Jessica J. O'Konek、Seok Ki ChoiDOI:10.1039/c9ob00503j日期:——
Design and
in vitro validation of polar α-nucleophile scaffolds that offer potent catalytic reactivity and practical utility for organophosphate decontamination.设计和体外验证极性α-亲核试剂支架,提供强大的催化活性和实际用于有机磷去污的效用。
表征谱图
-
氢谱1HNMR
-
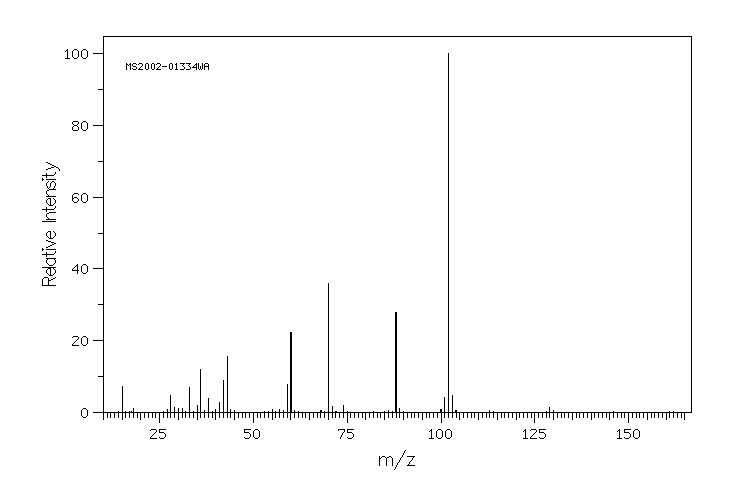
质谱MS
-
碳谱13CNMR
-
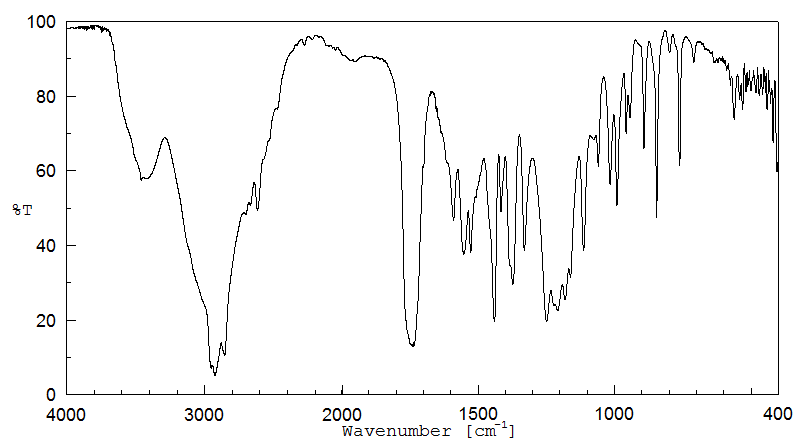
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸