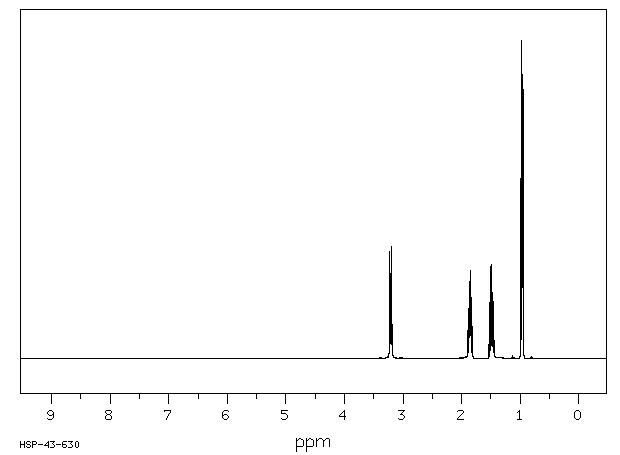
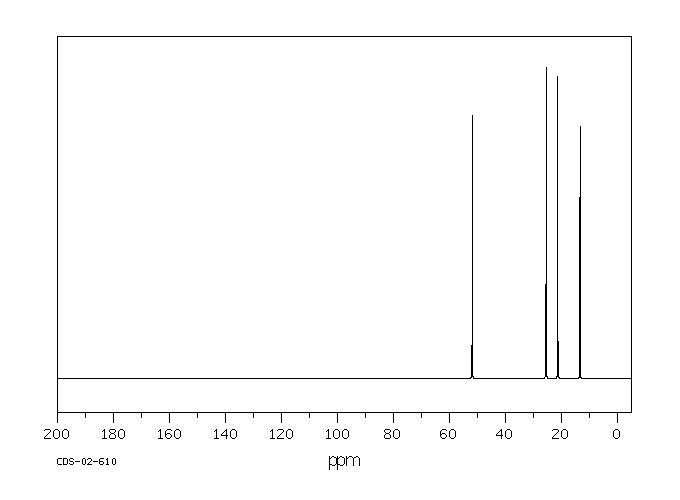
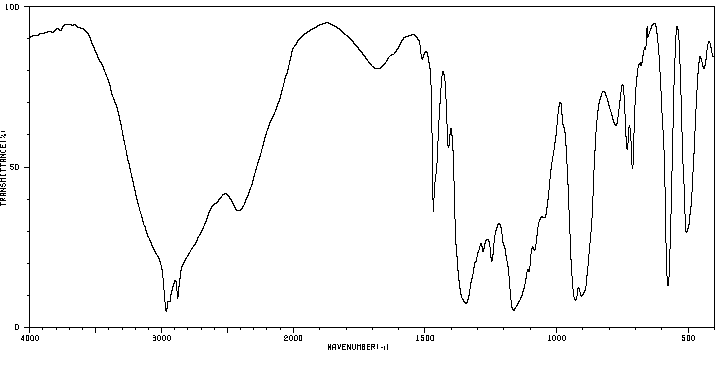
| Name: | 1-Butanesulfonic Acid (Pract) 99% (Titr.) Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 2386-47-2 |
Section 1 - Chemical Product MSDS Name:1-Butanesulfonic Acid (Pract) 99% (Titr.) Material Safety Data Sheet
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS | CAS# | Chemical Name | content | EINECS# |
| 2386-47-2 | 1-Butanesulfonic Acid (Pract) | 99 | 219-198-7 |
Hazard Symbols: C
Risk Phrases: 34
Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Causes burns.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. The toxicological properties of this substance have not been fully investigated. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. The toxicological properties of this substance have not been fully investigated.
Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES Eyes: Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Keep container tightly closed. Do not get on skin or in eyes. Do not ingest or inhale. Discard contaminated shoes.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2386-47-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid
Color: colorless
Odor: sulfurous odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 120 deg C ( 248.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: 120 deg C
Solubility in water: Slightly soluble.
Specific Gravity/Density: 1.1850g/cm3
Molecular Formula: C4H10O3S
Molecular Weight: 138.19
Section 10 - STABILITY AND REACTIVITY Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION RTECS#:
CAS# 2386-47-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Butanesulfonic Acid (Pract) - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION IATA
Shipping Name: ALKYLSULPHONIC ACIDS, LIQUID
Hazard Class: 8
UN Number: 2586
Packing Group: III
IMO
Shipping Name: ALKYLSULPHONIC ACIDS, LIQUID
Hazard Class: 8
UN Number: 2586
Packing Group: III
RID/ADR
Shipping Name: ALKYLSULPHONIC ACIDS, LIQUID
Hazard Class: 8
UN Number: 2586
Packing group: III
Section 15 - REGULATORY INFORMATION European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 34 Causes burns.
Safety Phrases:
S 25 Avoid contact with eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 36/37/39 Wear suitable protective clothing, gloves
and eye/face protection.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2386-47-2: No information available.
Canada
CAS# 2386-47-2 is listed on Canada's NDSL List.
CAS# 2386-47-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2386-47-2 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION N/A