(Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione | 6320-51-0
中文名称
——
中文别名
——
英文名称
(Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione
英文别名
5-(4-methoxy-benzylidene)-thiazolidine-2,4-dione;5-(4-Methoxybenzylidene)-1,3-thiazolidine-2,4-dione;(5Z)-5-[(4-methoxyphenyl)methylidene]-1,3-thiazolidine-2,4-dione
CAS
6320-51-0
化学式
C11H9NO3S
mdl
——
分子量
235.263
InChiKey
VRUKGUBMRBLJJW-TWGQIWQCSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.09
-
拓扑面积:80.7
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险性防范说明:P261,P264,P270,P271,P280,P301+P312,P302+P352,P304+P340,P305+P351+P338,P330,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H302,H315,H319,H335
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-硫代-5-(4-甲氧基苄亚基)噻唑烷-4-酮 5-(4-methoxybenzylidene)-2-thioxo-4-thiazolidinone 5462-97-5 C11H9NO2S2 251.33 —— (5Z)-5-(4-methoxybenzylidene)-2-thioxothiazolidin-4-one 81154-17-8 C11H9NO2S2 251.33 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (Z)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetamide 1039558-85-4 C13H12N2O4S 292.315 —— (Z)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetic acid —— C13H11NO5S 293.3 —— (Z)-5-(4-methoxybenzylidene)-4-thioxothiazolidin-2-one —— C11H9NO2S2 251.33 —— 3-benzyl-5-[(Z)-(4-methoxyphenyl)methylidene]-1,3-thiazolidine-2,4-dione —— C18H15NO3S 325.388 —— methyl 2-[(5Z)-5-[(4-methoxyphenyl)methylidene]-2,4-dioxo-1,3-thiazolidin-3-yl]acetate 614737-21-2 C14H13NO5S 307.327 —— 3-(4'-bromobenzyl)-5-(4'-methoxybenzylidene)thiazolidine-2,4-dione —— C18H14BrNO3S 404.284 —— (Z)-ethyl 2-(2-(5(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)acetamido)acetate 1280669-72-8 C17H18N2O6S 378.406 —— 4-[((Z)-5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)methyl]benzoic acid 1347755-15-0 C19H15NO5S 369.398 —— (Z)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)-N-phenylacetamide 1280669-94-4 C19H16N2O4S 368.413 —— (Z)-2-(5-(4-methoxybenzylidene)-2,4-dioxothiazolidin-3-yl)-N-(thiazol-2-yl)acetamide —— C16H13N3O4S2 375.429
反应信息
-
作为反应物:描述:(Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione 在 吡啶 、 盐酸 、 锂硼氢 、 potassium carbonate 、 溶剂黄146 作用下, 以 四氢呋喃 、 丙酮 为溶剂, 反应 25.5h, 生成 2-(5-(4-methoxybenzyl)-2,4-dioxothiazolidin-3-yl)acetic acid参考文献:名称:In vitro aldose reductase inhibitory activity of 5-benzyl-2,4-thiazolidinediones摘要:Several 5-benzyl-2,4-thiazolidinediones (5-7) were synthesised and tested as in vitro aldose reductase (ALR2) inhibitors. Most of them particularly N-unsubstituted 5-benzyl-2,4-thiazolidinediones 5 and (5-benzyl-2,4-dioxothiazolidin-3-yl)acetic acids 7, displayed moderate to high inhibitory activity levels. In detail, the insertion of an acetic chain on N-3 significantly enhanced ALR2 inhibitory potency, leading to acids 7 which proved to be the most effective among the tested compounds. In addition, in N-unsubstituted derivatives 5 the presence of an additional aromatic ring on the 5-benzyl moiety was generally beneficial. In fact, the ALR2 inhibition results of compounds 5-7, compared to those of the previously assayed corresponding 5-arylidene-2,4-thiazolidinediones, indicated that N-unsubstituted derivatives 5b, c and d, which bore an additional aromatic group in the para position of the 5-benzyl residue were significantly more effective than their 5-arylidene counterparts; in all other cases, the saturation of the exocyclic double bond C=C in 5 brought about a moderate decrease in activity. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2005.08.056
-
作为产物:描述:对氟苯甲醛 在 哌啶 、 potassium tert-butylate 、 溶剂黄146 作用下, 以 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 12.0h, 生成 (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione参考文献:名称:Structure–activity requirements for the antiproliferative effect of troglitazone derivatives mediated by depletion of intracellular calcium摘要:Depletion of calcium from the endoplasmic reticulum has shown to affect protein synthesis and cell proliferation. The anticancer effect of troglitazone was reported to be mediated by depletion of intracellular calcium stores resulting in inhibition of translation initiation. The unsaturated form of troglitazone displays similar anticancer properties in vitro. In this letter, we report our findings on the minimum structural requirements for both compounds to retain their calcium release and antiproliferative activities. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2004.02.087
文献信息
-
Novel ligands for the hisb10 zn2+ sites of the r-state insulin hexamer
-
[EN] PHAMACEUTICAL PREPARATIONS COMPRISING INSULIN<br/>[FR] PREPARATIONS PHARMACEUTIQUES CONTENANT DE L'INSULINE申请人:NOVO NORDISK AS公开号:WO2006005683A1公开(公告)日:2006-01-19Novel preparations comprising ligands for the HisB10 Zn2+ sites of the R-state insulin hexamer wherein the ligand is extended by protamine that are capable of prolonging the action of insulin preparations.
-
Stabilised insulin compositions申请人:Kaarsholm Christian Niels公开号:US20050065066A1公开(公告)日:2005-03-24The present invention provides pharmaceutical compositions comprising insulin and novel ligands for the His B10 Zn 2+ sites of the R-state insulin hexamer. The resulting preparations have improved physical and chemical stability.
-
Diversity-Oriented Synthesis of Spiropyrrolo[1,2-<i>a</i>]isoquinoline Derivatives via Diastereoselective and Regiodivergent Three-Component 1,3-Dipolar Cycloaddition Reactions:<i>In Vitro</i>and<i>in Vivo</i>Evaluation of the Antidiabetic Activity of Rhodanine Analogues作者:Amani Toumi、Sarra Boudriga、Khaled Hamden、Ismail Daoud、Moheddine Askri、Armand Soldera、Jean-Francois Lohier、Carsten Strohmann、Lukas Brieger、Michael KnorrDOI:10.1021/acs.joc.1c01544日期:2021.10.1endured retro-1,3-dipolar cycloaddition/recycloaddition reactions under thermal or catalytic conditions to regenerate the corresponding regioisomeric counterpart. In addition, DFT calculations were performed at the M062X/6-31++g(d,p) level of theory to unravel the origin of the reversal of regioselectivity and endo-stereoselectivity of the title 1,3-dipolar cycloaddition reactions. Upon treatment of Isatin开发了一种有效的非对映选择性路线,通过( Z )-5-亚芳基-1,3的一锅三组分 [3 + 2] 环加成反应获得新型螺吡咯[1,2- a ] 异喹啉-羟吲哚骨架-噻唑烷-2,4-二酮、靛红衍生物和 1,2,3,4-四氢异喹啉 (THIQ)。有趣的是,该反应的区域选择性既取决于温度,也取决于溶剂,允许以优异的产率合成两种区域异构的内-二螺吡咯并[2,1- a ]异喹啉并吲哚。前所未有地,每个异构体二螺吡咯并[2,1- a ]异喹啉并吲哚都经受了逆1,3-偶极环加成/再环加成反应在热或催化条件下再生相应的区域异构体对应物。此外,在 M062X/6-31++g(d,p) 理论水平上进行了 DFT 计算,以解开标题 1,3-偶极环加成反应的区域选择性和内立体选择性逆转的起源。在用 ( Z )-4-arylidene-5-thioxo-thiazolidin-2-ones 作为偶极体处理靛红、THIQ
-
Phosphoryl Chloride Mediated Synthesis of 5-Arylidene-2,4-Thiazolidinediones Derivatives via Aromatic Bisulfite Adducts作者:Sandeep Mohanty、Sandeep G、Arun KarmakarDOI:10.2174/15701786113106660081日期:2014.2The carbon-carbon bond formation by the condensation of bisulfite adduct of aromatic aldehydes with thiazolidine-2, 4-dione to furnish 5-arylidene-2,4-thiazolidinedione’s has been investigated. This novel methodology was applied to convert substituted aryl bisulfite adducts to corresponding 5-arylidene-2,4-thiazolidinedione’s with POCl3 in less-polar solvents such as toluene, chlorobenzene and o-xylene. 5-(4-methoxybenzylidene)thiazolidine-2,4-dione and 5-(4-ethoxybenzylidene)thiazolidine-2,4-dione were obtained in good yields.
表征谱图
-
氢谱1HNMR
-
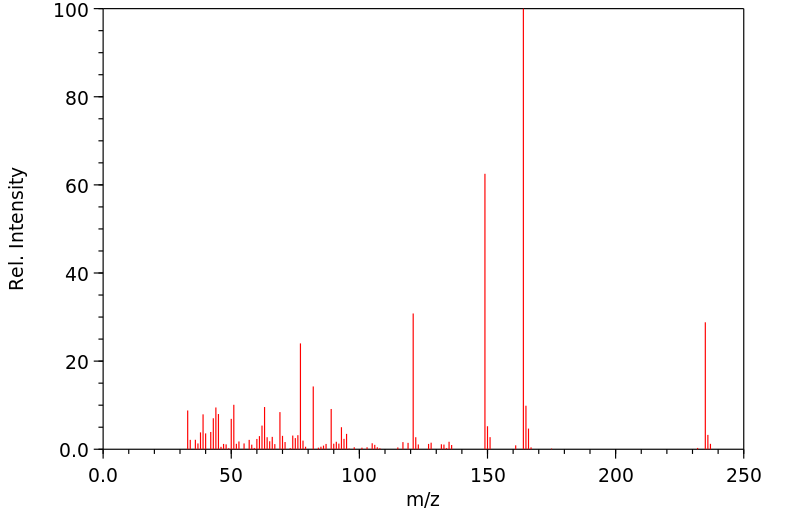
质谱MS
-
碳谱13CNMR
-
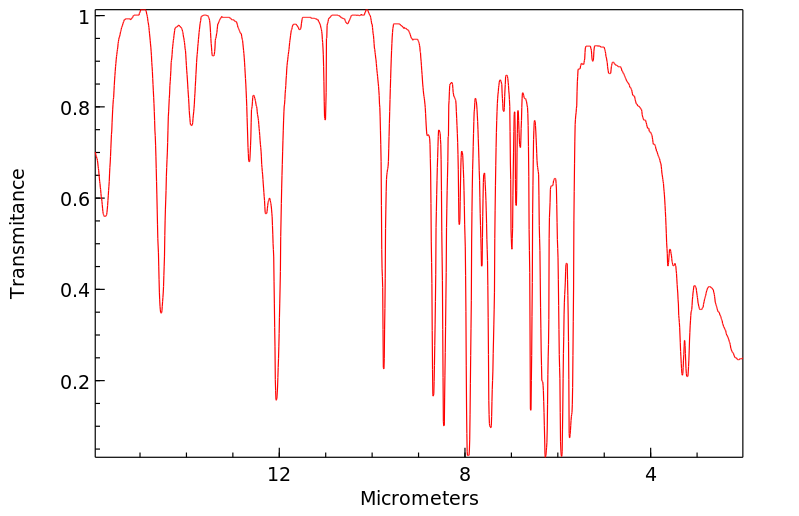
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯