2-氧代-1,2-二氢-3-吡啶羧酸甲酯 | 10128-91-3
中文名称
2-氧代-1,2-二氢-3-吡啶羧酸甲酯
中文别名
2-氧代-1,2-二氢-3-吡啶甲酸甲酯
英文名称
methyl 2-hydroxynicotinate
英文别名
2-hydroxynicotinic acid methyl ester;methyl 2-oxo-1H-pyridine-3-carboxylate
CAS
10128-91-3
化学式
C7H7NO3
mdl
MFCD08275055
分子量
153.137
InChiKey
SILBTMNCGYLTOK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:115-118°C
-
沸点:367.6±42.0 °C(Predicted)
-
密度:1.263±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:55.4
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2933399090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:冷藏保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Methyl 2-hydroxynicotinate
Product Name:
Synonyms: Methyl 2-oxo-1,2-dihydro-3-pyridinecarboxylate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
Methyl 2-hydroxynicotinate
Ingredient name:
CAS number: 10128-91-3
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H7NO3
Molecular weight: 153.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Methyl 2-hydroxynicotinate
Product Name:
Synonyms: Methyl 2-oxo-1,2-dihydro-3-pyridinecarboxylate
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
Methyl 2-hydroxynicotinate
Ingredient name:
CAS number: 10128-91-3
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels, refrigerated.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H7NO3
Molecular weight: 153.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2-氧代-1,2-二氢-3-吡啶羧酸甲酯是一种广泛应用于有机合成和医药行业的中间体。它主要被用于实验室的研发过程以及化工生产的各个环节中。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-羟基烟酸 2-Hydroxynicotinic acid 609-71-2 C6H5NO3 139.111 2-氨基吡啶-3-甲酸甲酯 methyl 2-aminonicotinate 14667-47-1 C7H8N2O2 152.153 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氧代-5-碘-1,2-二氢-3-吡啶甲酸甲酯 methyl 2-hydroxy-5-iodopyridine-3-carboxylate 116387-40-7 C7H6INO3 279.034 5-溴-2-羟基烟酸甲酯 methyl 5-bromo-2-oxo-1,2-dihydropyridine-3-carboxylate 120034-05-1 C7H6BrNO3 232.034 1-甲基-2-氧-1,2-二氢吡啶-3-羧酸甲酯 methyl 1-methyl-2-oxo-1,2-dihydropyridine-3-carboxylate 67367-27-5 C8H9NO3 167.164 1-甲基-2-氧代-1,2-二氢吡啶-3-羧酸 1-methyl-2-oxo-1,2-dihydropyridine-3-carboxylic acid 15506-18-0 C7H7NO3 153.137 —— methyl 1-(difluoromethyl)-2-oxo-1,2-dihydropyridine-3-carboxylate —— C8H7F2NO3 203.145 3-(羟基甲基)-2(1H)-吡啶酮 3-(hydroxymethyl)pyridin-2(1H)-one 42463-41-2 C6H7NO2 125.127 —— 1-(difluoromethyl) -2-oxo-1,2-dihydropyridine-3-carboxylic acid 1129458-32-7 C7H5F2NO3 189.118 —— methyl 2-oxo-1-phenyl-1,2-dihydropyridine-3-carboxylate 868171-80-6 C13H11NO3 229.235 —— Methyl 2-oxo-1-[(pyridin-3-yl)methyl]-1,2-dihydropyridine-3-carboxylate 1206454-47-8 C13H12N2O3 244.25 —— methyl 1-(4-fluorobenzyl)-2-oxo-1,2-dihydropyridine-3-carboxylate 888721-12-8 C14H12FNO3 261.253 —— 1-(2-hydroxyethyl)-2-oxo-1,2-dihydropyridine-3-carboxylic acid 954225-78-6 C8H9NO4 183.164 —— methyl 1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxylate 868171-66-8 C13H10FNO3 247.226 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:2-(烷基氨基)烟酸及其类似物。强大的血管紧张素II拮抗剂。摘要:人们发现,通过-CH2-NR'-连接(1)连接到联苯四唑的一系列吡啶和其他六元环杂环是有效的血管紧张素II拮抗剂。在嘧啶羧酸系列中(W = CR,X = N,Y = CH,Z = COOH),环外氮上带有烷基(R')的化合物比带有烷基(R)的化合物更有效在杂环上。相应的吡啶,哒嗪,吡嗪和1,2,4-三嗪羧酸也显示出有效的体外血管紧张素II拮抗作用。吡啶(W,X,Y = CH,Z = COOH,R'= n-C3H7)具有很强的体外活性(pA2 = 10.10,兔主动脉,Ki = 0.61 nM,大鼠肝脏中的受体结合)以及出色的口服降压活性和生物利用度。DOI:10.1021/jm00070a012
-
作为产物:描述:参考文献:名称:具有手性6,7-反式-双取代-1,4-恶唑烷的支架的周边选择性去甲肾上腺素再摄取抑制剂的实用合成的发展。摘要:描述了具有手性6,7-反式-二取代-1,4-氧杂庚烷作为新型支架的外围选择性去甲肾上腺素再摄取抑制剂的实用合成。具有优异的立体化学的氨基醇是通过优异的dr和ee获得的,它是通过Morita-Baylis-Hillman反应,Michael加成,马来酸盐的分离,还原和与(+)-的非对映异构体盐的形成,从市场上可买到的醛中获得的。 10-樟脑磺酸。在合成的早期阶段获得的所需单一立体异构体在完全伸缩的过程中用于七元环的形成,从而有效地提供了手性6,7-反式-二取代-1,4-氧杂庚烷。除了控制手性1,4-恶唑烷的dr和ee以及控制中间体甲磺酸酯与吡啶酮衍生物在S N 2反应中的N,O选择性,找到适合分离和纯度提高的合适中间体,从而可以实现无手性HPLC分离,无柱色谱的药物候选物的实用合成。优良的化学和光学纯度,总收率更高。DOI:10.1021/acs.oprd.7b00313
-
作为试剂:描述:2-[2-(4-chloro-phenyl)-2,4,5,6-tetrahydro-cyclopentapyrazol-3-yl]-2,N-dicyclohexyl-acetamide 、 potassium carbonate 、 methanesulfonic acid 2-[2-(4-chloro-phenyl)-2,4,5,6-tetrahydro-cyclopentapyrazol-3-yl]-2-cyclohexyl-ethyl ester 、 柠檬酸 在 2-氧代-1,2-二氢-3-吡啶羧酸甲酯 、 乙酸乙酯 、 Brine 、 Sodium sulfate-III 、 silica gel 、 ethyl acetate n-hexane 作用下, 以 N,N-二甲基甲酰胺 、 乙酸乙酯 为溶剂, 反应 12.25h, 以to give the title compound (60.2 mg, 0.12 mmol; 67%) as off-white solid的产率得到6-{2-[2-(4-chloro-phenyl)-2,4,5,6-tetrahydro-cyclopentapyrazol-3-yl]-2-cyclohexyl-ethoxy}-nicotinic acid methyl ester参考文献:名称:Cyclopentyl- and cycloheptylpyrazoles摘要:本发明涉及一种新型的环戊基和环庚基吡唑衍生物,其化学式为I,其中A和R1至R4如说明书和权利要求中所定义,以及其生理上可接受的盐。本发明还涉及包括这些化合物的组合物和使用这些化合物的方法。公开号:US08252826B2
文献信息
-
Direct Pd(II)-Catalyzed Site-Selective C5-Arylation of 2-Pyridone Using Aryl Iodides作者:Saurabh Maity、Debapratim Das、Souradip Sarkar、Rajarshi SamantaDOI:10.1021/acs.orglett.8b02112日期:2018.9.7transformation was highly regioselective and accomplished with a wide scope and functional group tolerance. Silver nitrate played a crucial role in this direct site-selective arylation. The method was extended to synthesize biologically active molecules.
-
[EN] Amide compounds and uses thereof<br/>[FR] COMPOSÉS AMIDES ET LEURS UTILISATIONS申请人:HUTCHISON MEDIPHARMA LTD公开号:WO2021197276A1公开(公告)日:2021-10-07Provided herein are novel amide compounds of formula (I), pharmaceutical compositions comprising same, methods for preparing same, and uses thereof, wherein the definition of each symbol is as described in the description.本文提供了化合物的新型酰胺化合物的公式(I),包括相同的药物组合物,制备相同的方法以及它们的用途,其中每个符号的定义如描述中所述。
-
Heterobicyclic pyrazole compounds and methods of use申请人:Blake F. James公开号:US20070238726A1公开(公告)日:2007-10-11Compounds of Formulas Ia and Ib, and stereoisomers, geometric isomers, tautomers, solvates, metabolites and pharmaceutically acceptable salts thereof, are useful for inhibiting receptor tyrosine kinases and for treating disorders mediated thereby. Methods of using compounds of Formula Ia and Ib, and stereoisomers, geometric isomers, tautomers, solvates and pharmaceutically acceptable salts thereof, for in vitro, in situ, and in vivo diagnosis, prevention or treatment of such disorders in mammalian cells, or associated pathological conditions are disclosed.化合物Ia和Ib的结构,以及其立体异构体、几何异构体、互变异构体、溶剂合物、代谢物和药学上可接受的盐,可用于抑制受体酪氨酸激酶并治疗由此介导的疾病。公开了使用化合物Ia和Ib的结构,以及其立体异构体、几何异构体、互变异构体、溶剂合物和药学上可接受的盐的方法,用于体外、体内和体内诊断、预防或治疗哺乳动物细胞中的这类疾病,或相关的病理条件。
-
MODIFIED COMPOUND OF ANDROGRAPHOLIDE申请人:Heilongjiang Zhenbaodao Pharmaceutical Co., Ltd.公开号:US20180346438A1公开(公告)日:2018-12-06The present disclosure discloses a modified compound of andrographolide, and particularly discloses a compound shown in formula (I) and (II) or a pharmaceutically acceptable salt thereof.本公开披露了穿心莲内酯的修改化合物,特别是公开了公式(I)和(II)所示的化合物或其药用可接受的盐。
-
[EN] POLYCYCLIC AMIDES AS UBE2K MODULATORS FOR TREATING CANCER<br/>[FR] AMIDES POLYCYCLIQUES UTILISÉS EN TANT QUE MODULATEURS D'UBE2K POUR LE TRAITEMENT DU CANCER申请人:BERG LLC公开号:WO2021138540A1公开(公告)日:2021-07-08Provided are compounds of Formula (I) and pharmaceutically acceptable salts and compositions thereof, which are useful for treating conditions associated with modulation of UBE2K.提供的是Formula (I)的化合物及其药用盐和组合物,可用于治疗与UBE2K调节相关的疾病。
表征谱图
-
氢谱1HNMR
-
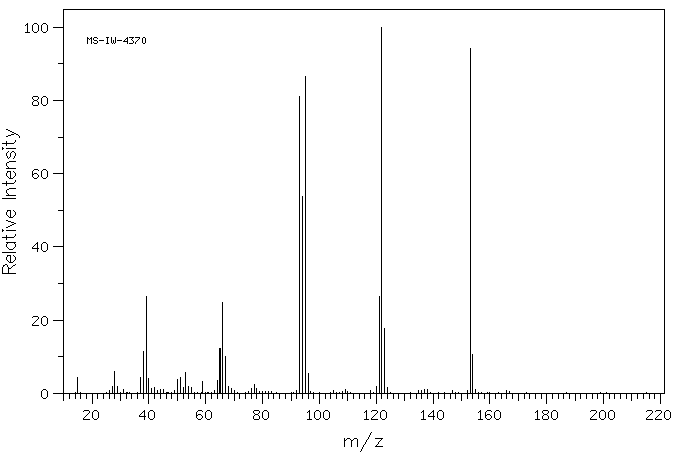
质谱MS
-
碳谱13CNMR
-
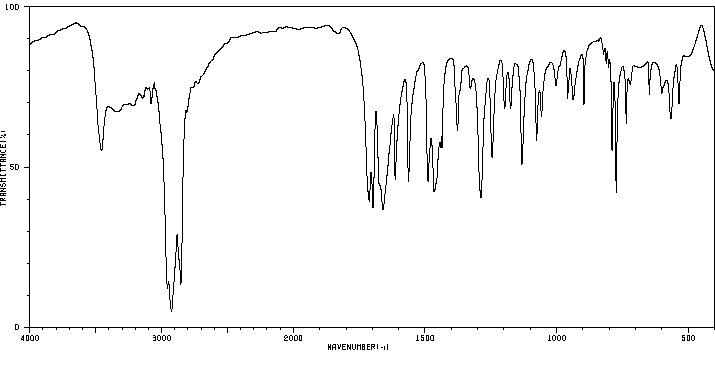
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-