3-氯-4-氟苯乙酮 | 2923-66-2
中文名称
3-氯-4-氟苯乙酮
中文别名
3'-氯-4'-氟苯乙酮(3-氯-4-氟苯乙酮);3'-氯-4'-氟苯乙酮
英文名称
3-chloro-4-fluoroacetophenone
英文别名
1-(3-chloro-4-fluorophenyl)ethanone;1-(3-chloro-4-fluorophenyl)ethan-1-one;3'-Chloro-4'-fluoroacetophenone
CAS
2923-66-2
化学式
C8H6ClFO
mdl
MFCD00042203
分子量
172.586
InChiKey
PCJPESKRPOTNGU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:42 °C
-
沸点:126 °C
-
密度:1.258±0.06 g/cm3(Predicted)
-
闪点:126-127°C/15mm
-
溶解度:溶于甲醇
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放在阴凉干燥处。
SDS
| Name: | 1-(3-Chloro-4-fluorophenyl)ethan-1-one 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2923-66-2 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2923-66-2 | 1-(3-Chloro-4-fluorophenyl)ethan-1-one | 97% | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
Causes respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2923-66-2: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 126 - 128 deg C @15mmHg
Freezing/Melting Point: 40 - 42 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6ClFO
Molecular Weight: 173
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents, amines.
Hazardous Decomposition Products:
Hydrogen chloride, chlorine, carbon monoxide, carbon dioxide, fluorine, hydrogen fluoride gas.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2923-66-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-(3-Chloro-4-fluorophenyl)ethan-1-one - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 2923-66-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2923-66-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2923-66-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法:主要用作农药和医药的中间体。
用途简介: 暂无具体内容。
用途: 主要用作农药和医药的中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯-4-氟苯甲酰氯 3-chloro-4-fluorobenzoyl chloride 65055-17-6 C7H3Cl2FO 193.005 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-Chloro-4-fluoro-I+/--oxobenzeneacetaldehyde 79784-35-3 C8H4ClFO2 186.57 2-溴-3′-氯-4′-氟苯乙酮 2-bromo-1-(3-chloro-4-fluorophenyl)ethanone 63529-30-6 C8H5BrClFO 251.483 —— 2-Chlor-4-ethyl-1-fluorbenzol 2338-49-0 C8H8ClF 158.603 1-(3-氯-4-氟苯基)-3-苯基丙烷-1,3-二酮 1-(3-chloro-4-fluorophenyl)-3-phenylpropane-1,3-dione 38448-97-4 C15H10ClFO2 276.695 —— 1-(3-chloro-4-fluorophenyl) butane-1,3-dione 38440-12-9 C10H8ClFO2 214.624 1-(3-氯-4-氟苯基)-3-(4-氟苯基)丙-2-烯-1-酮 4-fluorobenzal-3'-chloro-4'-fluoroacetophenone 113368-18-6 C15H9ClF2O 278.686 1-(3-氯-4-氟苯基)乙醇 1-(3-chloro-4-fluorophenyl)ethan-1-ol 878572-03-3 C8H8ClFO 174.602 —— 4,4,4-trifluoro-1-(3-chloro-4-fluorophenyl) butane-1,3-dione 38440-19-6 C10H5ClF4O2 268.595 3-氯-4-氟苯乙酸 2-(3-chloro-4-fluorophenyl)acetic acid 705-79-3 C8H6ClFO2 188.586 3-氯-4-氟苯甲酸 3-chloro-4-fluorobenzoic acid 403-16-7 C7H4ClFO2 174.559 —— ethyl 4-(3-chloro-4-fluorophenyl)-2,4-dioxobutanoate 549526-24-1 C12H10ClFO4 272.66 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:噻唑/噻二唑甲酰胺支架衍生物的设计、合成和生物学评价作为潜在的 c-Met 激酶抑制剂用于癌症治疗摘要:摘要 作为我们不断努力发现新型 c-Met 抑制剂作为抗肿瘤药物的一部分,我们设计、合成了四个系列的噻唑/噻二唑甲酰胺衍生类似物,并评估了其针对 c-Met 和四种人类癌细胞系的体外活性。经过五个周期的构效关系优化后,发现化合物51am在生化和细胞分析中都是最有前途的抑制剂。此外,51am对几种 c-Met 突变体表现出效力。从机制上讲,51am不仅诱导 MKN-45 细胞的细胞周期停滞和凋亡,而且抑制细胞和无细胞系统中的 c-Met 磷酸化。它还在 BALB/c 小鼠中表现出良好的药代动力学特征。此外, 51am与 c-Met 和 VEGFR-2 的结合模式为选择性 c-Met 抑制剂的发现提供了新的见解。总而言之,这些结果表明51am可能是值得进一步开发的抗肿瘤候选药物。DOI:10.1080/14756366.2023.2247183
-
作为产物:描述:参考文献:名称:2-Hydroxymethyl-4-[5-(4-methoxyphenyl)-3-trifluoromethyl-1H-1-pyrazolyl]-1-benzenesulfonamide (DRF-4367): an orally active COX-2 inhibitor identified through pharmacophoric modulation摘要:在N1位含有新颖药效团的1,5-二芳基吡唑类类似物被设计、合成并对体外环氧合酶(COX-1/COX-2)抑制活性进行了评估。在C-5苯环的4位及其周围的变异与C-3位的CF3和CHF2基团结合,展现出高度的效力和选择性指数(SI),用于COX-2抑制。这些强效化合物的体内评估与先前的一些化合物相比,显示4-OMe-苯基类似物6和4-NHMe-苯基类似物9(C-3位含CF3),以及4-OEt-苯基类似物19(C-3位含CHF2)对COX-2的抑制效力超过了塞来昔布。除了出色的抗炎、解热、镇痛和抗关节炎特性外,化合物6(DRF-4367)被发现具有优秀的药代动力学特性,长期关节炎研究中的胃肠道安全性,以及在人体全血检测中的COX-2效力。因此,化合物6被选为口服活性的抗炎候选物进行临床前评估。DOI:10.1039/b402787f
文献信息
-
Cell adhesion-inhibiting antiinflammatory and immune-suppressive compounds申请人:Abbott Laboratories公开号:US20040116518A1公开(公告)日:2004-06-17The present invention relates to novel cinnamide compounds that are useful for treating inflammatory and immune diseases and cerebral vasospasm, to pharmaceutical compositions containing these compounds, and to methods of inhibiting inflammation or suppressing immune response in a mammal.本发明涉及新型肉桂酰胺化合物,用于治疗炎症和免疫性疾病以及脑血管痉挛,以及含有这些化合物的药物组合物,以及在哺乳动物中抑制炎症或抑制免疫反应的方法。
-
Structure-activity relationship of new antimalarial 1-aryl-3-susbtituted propanol derivatives: Synthesis, preliminary toxicity profiling, parasite life cycle stage studies, target exploration, and targeted delivery作者:Miguel Quiliano、Adriana Pabón、Ernest Moles、Leonardo Bonilla-Ramirez、Isabelle Fabing、Kim Y. Fong、Diego A. Nieto-Aco、David W. Wright、Juan C. Pizarro、Ariane Vettorazzi、Adela López de Cerain、Eric Deharo、Xavier Fernández-Busquets、Giovanny Garavito、Ignacio Aldana、Silvia GalianoDOI:10.1016/j.ejmech.2018.04.038日期:2018.5Design, synthesis, structure-activity relationship, cytotoxicity studies, in silico drug-likeness, genotoxicity screening, and in vivo studies of new 1-aryl-3-substituted propanol derivatives led to the identification of nine compounds with promising in vitro (55, 56, 61, 64, 66, and 70-73) and in vivo (66 and 72) antimalarial profiles against Plasmodium falciparum and Plasmodium berghei. Compounds
-
Identification of benzothiazones containing a hexahydropyrrolo[3,4-<i>c</i>]pyrrol moiety as antitubercular agents against MDR-MTB作者:Xican Ma、Bing Han、Aoyu Wang、Lu Yang、Menghao Huang、Kushan Chowdhury、Jian Gu、Kai Zhang、Kai LvDOI:10.1039/d0ra00750a日期:——
IMB1603 , a spiro-benzothiazone compound discovered by our lab, displayed potent anti-MTB activityin vitro andin vivo . In this study, benzothiazones containing a hexahydropyrrolo[3,4-c ]pyrrol moiety were synthesized and evaluated based onIMB1603 . -
[EN] HETEROCYCLIC CARBOXYLATE COMPOUNDS AS GLYCOLATE OXIDASE INHIBITORS<br/>[FR] CARBOXYLATES HÉTÉROCYCLES EN TANT QU'INHIBITEURS DE GLYCOLATE OXYDASE申请人:GYANRX SCIENCES INC公开号:WO2021086874A1公开(公告)日:2021-05-06The present disclosure relates generally to modulators of human glycolate oxidase enzyme and methods of use and manufacture thereof. (I)本公开涉及人类乙醇酸氧化酶酶的调节剂及其使用和制造方法。
-
A Mechanochemical Zinc-Mediated Barbier-Type Allylation Reaction under Ball-Milling Conditions作者:JieXiang Yin、Roderick T. Stark、Ian A. Fallis、Duncan L. BrowneDOI:10.1021/acs.joc.9b02876日期:2020.2.21A ball-milling-enabled zinc-mediated Barbier-type allylation reaction is reported. Notably, running the reaction in this manner renders it effective irrespective of the initial morphology of the zinc metal. The process is operationally simple, does not require inert atmospheres or dry solvents, and is reported over a range of aldehyde and ketone substrates; a gram-scale process is demonstrated.
表征谱图
-
氢谱1HNMR
-
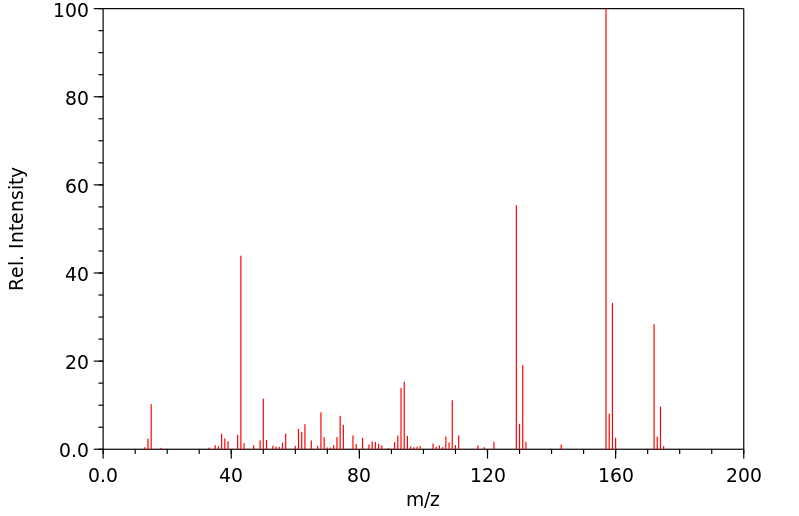
质谱MS
-
碳谱13CNMR
-
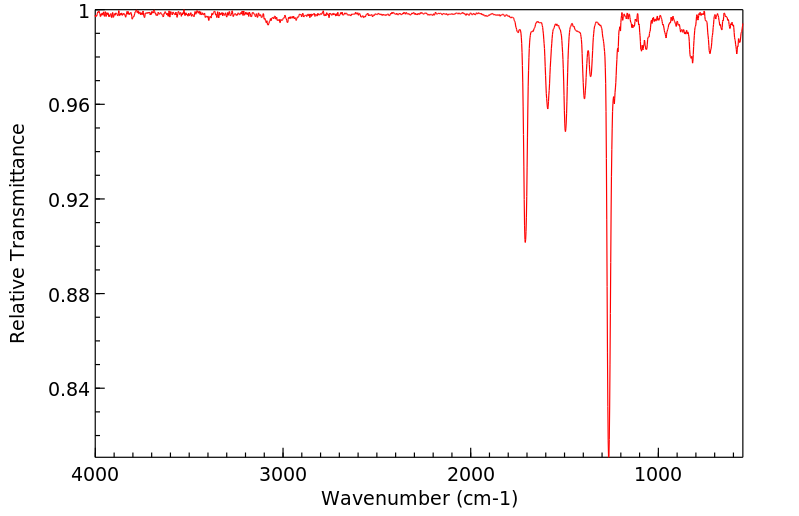
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷