2,6-Dimethyl-octa-1,3t,7-trien | 51703-69-6
中文名称
——
中文别名
——
英文名称
2,6-Dimethyl-octa-1,3t,7-trien
英文别名
2,6-dimethylocta-1,3,7-triene;2,6-Dimethylocta-1,3,7-trien;isomyocorene;2,6-dimethyl-octa-1,3t,7-triene;2,6-dimethyl-1,3,7-octatriene;2,6-Dimethyl-1,3-trans-7-octatrien;(3E)-2,6-dimethylocta-1,3,7-triene
CAS
51703-69-6
化学式
C10H16
mdl
——
分子量
136.237
InChiKey
JWMIAIJDECOUIA-VOTSOKGWSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:165.6±10.0 °C(Predicted)
-
密度:0.767±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:10
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:由异戊二烯合成(+)-或(-)-香茅醇摘要:(+)-或(-)-香茅醇是从异戊二烯开始的三到四个步骤合成的。DOI:10.1039/c39750000170
-
作为产物:描述:天然橡胶 在 bis(tributylphosphine)dichloropalladium(II) 作用下, 以 甲醇 、 异丙醇 为溶剂, 生成 2,6-Dimethyl-octa-1,3t,7-trien参考文献:名称:由异戊二烯合成(+)-或(-)-香茅醇摘要:(+)-或(-)-香茅醇是从异戊二烯开始的三到四个步骤合成的。DOI:10.1039/c39750000170
文献信息
-
Facile synthesis of (�)-2,6-dimethyloctan-1-ol formate, the biologically active analog of the smaller flour beetle aggregation pheromone, from 1-allyloxy- and 1-benzyloxy-2,6-dimethyl-2,7-octadienes, the telomers of isoprene with allyl and benzyl alcohols作者:L. I. Zakharkin、V. V. Guseva、P. V. PetrovskiiDOI:10.1007/bf00714434日期:1995.8of isoprene with allyl, benzyl, 2-chloroethyl and methyl alcohols on π-allylpalladium complex catalysts was carried out. The structures of the obtained telomers were determined by1H NMR spectroscopy. 1-Alkoxy-2,6-dimethyl-2,7-octadiene is a predominant telomer. 2,6-Dimethyloctan-1-ol, which reacts with HCOOH to give (±)-2,6-dimethyloctan-1-ol formate in high yield, was obtained from 1-allyloxy- and
-
Dimerization of Isoprene by Palladium-Diphosphine Complex Catalyst作者:Kuniyuki Takahashi、Go Hata、Akihisa MiyakeDOI:10.1246/bcsj.46.600日期:1973.2tail-to-tail dimerization almost selectively, while the addition of a large amount of phenol (phenol/isoprene=1/1.5) afforded head-to-head dimers (2-vinyl-5-methyl-1,6-heptadiene and 3,6-dimethyl-1,cis-3,7-octatriene) as main dimers (more than 40% of all the dimers). In a 1:3 ratio of phenol to isoprene the dimers consisted of mainly 2,6-dimethyl-1,trans-3,7-octatriene derived from a head-to-tail dimerization研究了 PdBr2(Ph2PCH2CH2PPh2)2-PhONa 催化剂催化异戊二烯的低聚反应。苯酚的加入大大提高了催化剂的活性。鉴定了五个线性二聚体。苯酚与异戊二烯的摩尔比显着影响异构二聚体的分布。在少量苯酚(苯酚/异戊二烯 = 1/30)存在下的反应几乎选择性地从尾对尾二聚反应中得到 2,7-二甲基-1,反式-3,7-辛三烯,而添加大量苯酚(苯酚/异戊二烯=1/1.5)得到头对头二聚体(2-乙烯基-5-甲基-1,6-庚二烯和3,6-二甲基-1,顺-3, 7-辛三烯)作为主要二聚体(占所有二聚体的 40% 以上)。在苯酚与异戊二烯的比例为 1:3 时,二聚体主要由 2,6-二甲基-1,反式-3,7-辛三烯组成,源自头尾二聚。
-
Telomerization and dimerization of isoprene by in situ generated palladium–carbene catalysts作者:Ralf Jackstell、Anne Grotevendt、Dirk Michalik、Larbi El Firdoussi、Matthias BellerDOI:10.1016/j.jorganchem.2007.06.039日期:2007.10The palladium-catalyzed telomerization of isoprene with methanol and dimerization of isoprene have been studied in presence of in situ generated palladium–carbene catalysts. Unprecedented catalyst productivity has been observed for these two reactions. A selectivity switch from the telomer to the dimer product occurred by using different substituted carbene ligands. Among the imidazolium salts tested
-
Preparation of diacyloxyalkadienes申请人:BASF Aktiengesellschaft公开号:US04480123A1公开(公告)日:1984-10-30A process for preparing diacyloxyalkadienes of the formula ##STR1## where R.sup.1 to R.sup.6 are each hydrogen or a hydrocarbon radical, which contain one or more hydrocarbon radicals having one or more non-conjugated double bonds, by reacting an aliphatic triene of the formula ##STR2## in which R.sup.1 to R.sup.6 have the same meanings given above with a carboxylic acid of the formula R.sup.7 -COOH in which R.sup.7 has the same meaning given above, in the presence of a catalyst which contains palladium, platinum or salts of these metals and in the presence of oxygen.
-
Descoins,C. et al., Bulletin de la Societe Chimique de France, 1971, p. 4087 - 4093作者:Descoins,C. et al.DOI:——日期:——
表征谱图
-
氢谱1HNMR
-
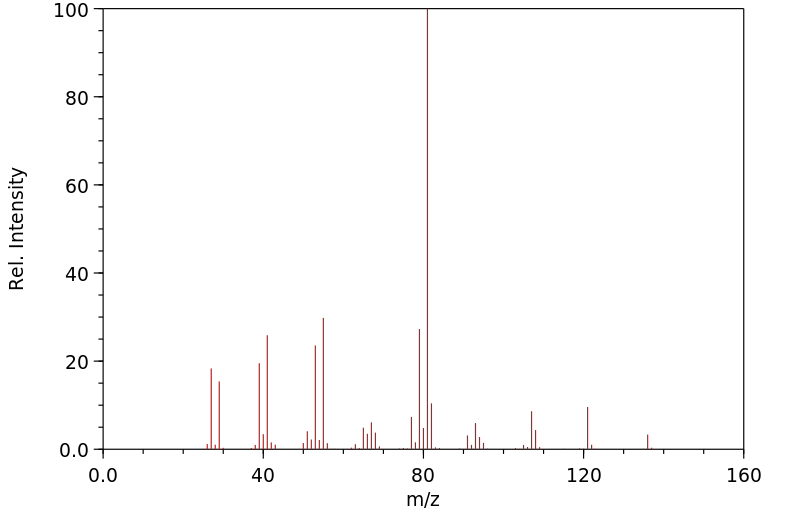
质谱MS
-
碳谱13CNMR
-
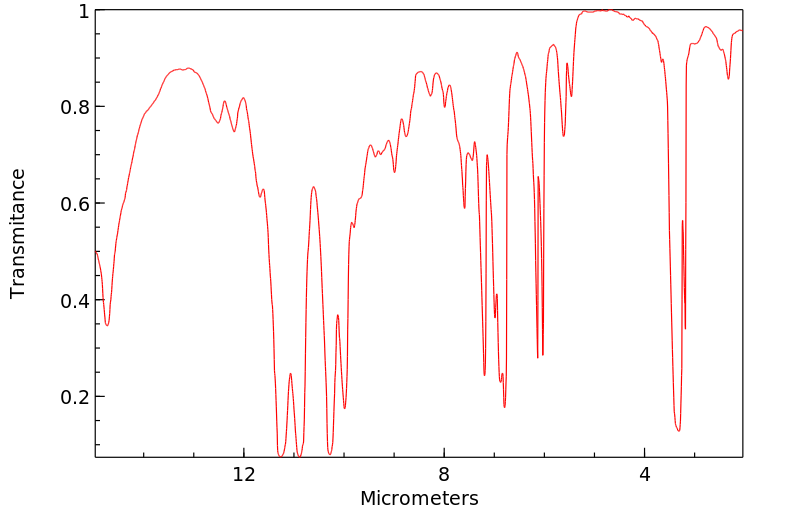
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-