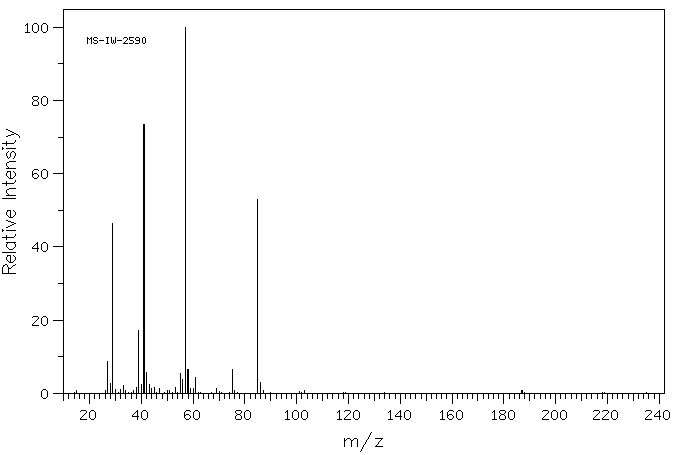
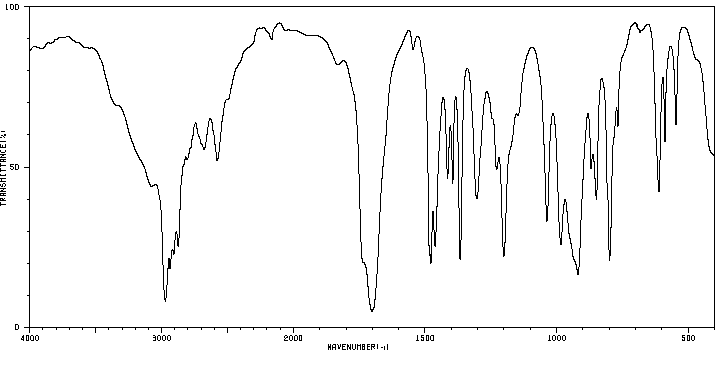
硫代特戊酸 | 55561-02-9
物质功能分类
中文名称
硫代特戊酸
中文别名
三甲基硫代乙酸
英文名称
thiopivalic acid
英文别名
2,2-dimethylpropanethioic S-acid;2-methyl-2-methylthiopropionic acid
CAS
55561-02-9
化学式
C5H10OS
mdl
MFCD00009729
分子量
118.2
InChiKey
JHSXHKMJBWOMRU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:125-127 °C
-
密度:0.966±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:18.1
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品运输编号:UN 2920
-
包装等级:II
-
危险类别:8
SDS
| Name: | Thiopivalic acid 97% Material Safety Data Sheet |
| Synonym: | Trimethylthiolacetic aci |
| CAS: | 55561-02-9 |
Synonym:Trimethylthiolacetic aci
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 55561-02-9 | Thiopivalic acid, 97% | 259-708-5 |
Risk Phrases: 10 35
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Causes severe burns.Stench.
Potential Health Effects
Eye:
Contact with eyes may cause severe irritation, and possible eye burns.
Skin:
May cause severe irritation and possible burns.
Ingestion:
Not available.
Inhalation:
May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung edema. Causes chemical burns to the respiratory tract.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Not available.
Storage:
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 55561-02-9: Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 125.0 - 127.0 deg C @ 760.00m
Freezing/Melting Point: 0 deg C
Autoignition Temperature: Not available.
Flash Point: 23 deg C ( 73.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9300g/cm3
Molecular Formula: C5H10OS
Molecular Weight: 118.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong oxidizing agents - strong bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 55561-02-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Thiopivalic acid, 97% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.*
Hazard Class: 8 (3)
UN Number: 2920
Packing Group: II
IMO
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.
Hazard Class: 8 (3)
UN Number: 2920
Packing Group: II
RID/ADR
Shipping Name: CORROSIVE LIQUID, FLAMMABLE, N.O.S.
Hazard Class: 8
UN Number: 2920
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 10 Flammable.
R 35 Causes severe burns.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 55561-02-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 55561-02-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 55561-02-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
有机中间体。
用途简介暂无相关信息。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— S-amino 2,2-dimethylpropanethioate —— C5H11NOS 133.214
反应信息
-
作为反应物:描述:参考文献:名称:杂狄尔斯-阿尔德与1,3-噻唑-5-(4反应ħ)-thiones摘要:Hetro- Diels - Alder与1,3-噻唑-5(4 H)-硫酮的反应DOI:10.1002/hlca.19880710825
-
作为产物:参考文献:名称:用劳森试剂将羧酸转化为硫代酸的简单方法摘要:已经开发出一种使用劳森试剂将羧酸转化为硫酯的一步方案。DOI:10.1016/j.tetlet.2009.09.080
文献信息
-
Asymmetric Desymmetrization of Oxetanes for the Synthesis of Chiral Tetrahydrothiophenes and Tetrahydroselenophenes作者:Renwei Zhang、Wengang Guo、Meng Duan、K. N. Houk、Jianwei SunDOI:10.1002/anie.201910917日期:2019.12.9Chiral tetrahydrothiophenes and tetrahydroselenophenes are highly useful structural units. Described here is a new catalytic asymmetric approach for their synthesis. With a suitable chiral Brønsted acid catalyst, an oxetane desymmetrization by a well-positioned internal sulfur or selenium nucleophile proceeded efficiently to generate all-carbon quaternary stereocenters with excellent enantioselectivities
-
Studies on topical antiinflammatory agents. II. Synthesis and vasoconstrictive activity of 21-substituted corticosteroids with sulfur-containing moieties.作者:Morihiro MITSUKUCHI、Tomoyuki IKEMOTO、Minoru TAGUCHI、Shohei HIGUCHI、Satoshi ABE、Hajime YASUI、Katsuo HATAYAMA、Kaoru SOTADOI:10.1248/cpb.37.1795日期:——As part of the search for new topical antiinflammatory agents, various 21-substituted corticosteroids having sulfur-containing moieties were prepared and tested for vasoconstrictive activity in humans. A structure-activity relationship study revealed that substitution of the 21-hydroxy group with a lower alkyl-thio group enhanced the activity. The activities of the 21-methylthio (3Ad) and the 21-ethylthio (3Ae) compounds were more potent than that of 9α-fluoro-11β, 21-dihydroxy-16β-methyl-17α-valeroyloxy-1, 4-pregnadiene-3, 20-dione (betamethasone 17-valerate, BV).
-
2,2-Disubstituted 4-Acylthio-3-oxobutyl Groups as Esterase- and Thermolabile Protecting Groups of Phosphodiesters作者:Emilia Kiuru、Zafar Ahmed、Harri Lönnberg、Leonid Beigelman、Mikko OraDOI:10.1021/jo302421u日期:2013.2.12-substituents and the size of the acylthio group. The acyl group evidently migrates from the sulfur atom to C3-gem-diol obtained by hydration of the keto group and the exposed mercapto group attacks on C1 resulting in departure of the protecting group as 4,4-disubstituted 3-acyloxy-4,5-dihydrothiophene with concomitant release of the desired phosphodiester.已经引入了五个不同的2,2-二取代的4-酰基硫基-3-氧代丁基作为酯酶不稳定的磷酸二酯保护基,它们另外是热不稳定的。的磷酸三酯1 - 3通过HPLC-ESI-MS制备,以确定在37酶和非酶去除这些基团的速率℃和pH 7.5。另外,1 H NMR光谱监测用于中间体和产物的结构表征。当用猪肝酯酶处理时,这些基团通过酶促脱酰基作用,然后快速化学环化成4,4-二取代的二氢噻吩-3(2 H)-一。可以通过4-酰硫基取代基的性质来调节酶促脱保护的速率,苯甲酰基和乙酰基的去除速度是新戊酰基的50和5倍。酶促脱保护后未观察到谷胱甘肽的烷基化。根据2-取代基的电负性和酰硫基的大小,非酶促脱保护的半衰期为0.57至35小时。酰基显然来自硫原子C3-迁移宝石由酮基的水合和产生保护基团的离去C1上的暴露的巯基的攻击得到二醇如4,4-二取代-3-酰氧基-4,5- -二氢噻吩并伴随释放所需的磷酸二酯。
-
A Facile Synthesis of Thioacids by Hydrolysis of 1-(Acylthio)ethaniminium Chlorides作者:Masaharu Toriyama、Haruo Kamijo、Shigeyasu Motohashi、Toshio Takido、Kunio ItabashiDOI:10.1080/10426500307837日期:2003.8.1A facile method for the preparation of thioacids in moderate to good yields has been developed by hydrolysis of 1-(acylthio)ethaniminium chlorides under a liquid-liquid two phase system consisting of benzene and a sodium hydroxide aqueous solution at room temperature. We have achieved facile preparation of these compounds without use of toxic compounds such as hydrogen sulfide.
-
Mononucleoside Phosphotriester Derivatives with S-Acyl-2-thioethyl Bioreversible Phosphate-Protecting Groups: Intracellular Delivery of 3'-Azido-2',3'-dideoxythymidine 5'-Monophosphate作者:Isabelle Lefebvre、Christian Perigaud、Alain Pompon、Anne-Marie Aubertin、Jean-Luc Girardet、Andre Kirn、Gilles Gosselin、Jean-Louis ImbachDOI:10.1021/jm00020a007日期:1995.9The synthesis, in vitro anti-HIV-1 activity, and decomposition pathways of several mononucleoside phosphotriester derivatives of 3'-azido-2',3'-dideoxythymidine (AZT) incorporating a new kind of carboxylate esterase-labile transient phosphate-protecting group, namely, S-acyl-2-thioethyl, are reported. All the described compounds showed marked antiviral activity in thymidine kinase-deficient CEM cells
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
硫代辛酸
硫代特戊酸
硫代棕榈酸
硫代乙酸钾
硫代乙酸
十四烷硫酸
三氟硫代乙酸
3-巯基丙酸
2-甲基硫代丙i酸
2-氯硫代戊S-酸
2-巯基硫代乙酸
2,2-二甲硫基丁酸
2,2-二氯硫代乙S-酸
Butylthioisobutyric acid
heneicosafluoro-n-decylthioacetic acid
tridecafluoro-n-hexylthioacetic acid
thioheptanoic acid
chlorodifluorothioacetic acid
methyl thioglycolate
3-isopropylthiopropionic acid
2-pentafluoroethylthiopropionic acid
3-pentafluoroethylthiopropionic acid
Propanethioic acid, 3-amino-3-imino-
3-Amino-monothiopropionsaeure
2,3-Dichlor-thiopropionsaeure
2-methoxy-2-methylpropanethioic acid
2-(tetradecylthio)thiolacetic acid
vinylthioacetate
cycloheptanecarbothioic S-acid
1,2-dithio-oxalic acid
2-Methyl-penten-(2)-thiosaeure-(5)
β-aminothiobutyric acid
(R*,R*)-2,3-Mercaptobutanedicarbothioic acid
β-Chlor-thioisobuttersaeure
Fluorchlor-thioessigsaeure
Monobromthioessigsaeure
methylenedithioacetic acid
thio-3-methylbutyric acid
Allylthioessigsaeure
1,10-dithio-decanedioic acid
1,9-dithio-nonanedioic acid
5H-Octafluorthiolvaleriansaeure
2,2-dichloro-3,3,3-trifluoropropanethioic S-acid
2,2-dimethyl-3-sulfanyl-3-sulfanylidenepropanethioic S-acid
Monothiooxalsaeure
ethoxythioacetic S-acid
1,8-dithio-octanedioic acid
thiononanoic acid
2-Methyl-hexen-(2)-thiosaeure-(6)