3-羟基丙酸甲酯 | 6149-41-3
中文名称
3-羟基丙酸甲酯
中文别名
——
英文名称
methyl ester (3-hydroxy) propionic acid
英文别名
methyl 3-hydroxypropionate;methyl 3-hydroxypropanoate
CAS
6149-41-3
化学式
C4H8O3
mdl
MFCD00272293
分子量
104.106
InChiKey
RVGLEPQPVDUSOJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:147-148 °C(Solv: benzene (71-43-2); cyclohexane (110-82-7))
-
沸点:179°C (estimate)
-
密度:1.1050
-
溶解度:可溶于氯仿、甲醇
计算性质
-
辛醇/水分配系数(LogP):-0.6
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.75
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
海关编码:2918199090
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Methyl 3-hydroxypropanoate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 3-hydroxypropanoate
CAS number: 6149-41-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H8O3
Molecular weight: 104.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Methyl 3-hydroxypropanoate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 3-hydroxypropanoate
CAS number: 6149-41-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C4H8O3
Molecular weight: 104.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 β-丙内酯 β-Propiolactone 57-57-8 C3H4O2 72.0636 3-羟基丙酸 3-hydroxypropionic acid 503-66-2 C3H6O3 90.0788 3-氯丙酸甲酯 methyl 3-chloropropionate 6001-87-2 C4H7ClO2 122.551 3-乙酰氧基丙酸甲酯 methyl β-acetoxypropionate 38003-42-8 C6H10O4 146.143 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 丙酸甲酯 Methyl propionate 554-12-1 C4H8O2 88.1063 3-羟基-2-甲基丙酸甲酯 methyl 3-hydroxy-2-methylpropanoate 42998-03-8 C5H10O3 118.133 3-乙酰氧基丙酸甲酯 methyl β-acetoxypropionate 38003-42-8 C6H10O4 146.143
反应信息
-
作为反应物:参考文献:名称:Kras-G12C抑制剂杂环化合物摘要:本申请涉及一类式I所示Kras‑G12C抑制剂杂环化合物,及其制备方法和该类化合物在肿瘤疾病,如肺癌、结直肠癌和胰腺癌等中的预防和治疗用途。在制备过程中,通过SN2反应、上保护、偶联反应、脱保户、缩合反应等一系列反应,得到通式化合物I。公开号:CN113004269A
-
作为产物:描述:参考文献:名称:Effect of deuteration on the metabolism and clearance of some pharmacologically active compounds-synthesis andin vitrometabolism of deuterated derivatives of dronedadrone摘要:描述了特定氘代衍生物多奈哌齐(dronedarone)一族的合成及其体外代谢研究。无论重标记的位置如何,母体化合物的代谢稳定性和清除率对氘取代并不敏感。DOI:10.1002/jlcr.3090
-
作为试剂:描述:乙醇 、 2-fluoro-3-p-bromophenylacrylic acid 在 fac-tris(2-phenylpyridinato-N,C2')iridium(III) 、 3-羟基丙酸甲酯 、 三乙胺 作用下, 以 乙腈 为溶剂, 生成参考文献:名称:CN116178148摘要:公开号:
文献信息
-
[EN] EPHA4 CYCLIC PEPTIDE ANTAGONISTS AND METHODS OF USE THEREOF<br/>[FR] ANTAGONISTES PEPTIDIQUES CYCLIQUES DE L'EPHA4 ET LEURS PROCÉDÉS D'UTILISATION申请人:IRON HORSE THERAPEUTICS INC公开号:WO2019213620A1公开(公告)日:2019-11-07Disclosed herein are compounds and methods of use thereof for the modulation of EphA4 receptor activity. In an aspect, is provided a method of treating or preventing a disease or disorder mediated by EphA4, comprising administering to a subject in need thereof a therapeutically effective amount of a compound as described herein, including certain embodiments, or the structural Formula (I), (l-A), (II), (III), (IV), (IV-1), (V), (Vl-A), (Vl-B), (VII-1), (VII-2), (VIII-1), or (VIII-2), or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, or an isotopic variant thereof; or a pharmaceutically acceptable salt, solvate, or hydrate thereof.本文揭示了化合物及其使用方法,用于调节EphA4受体活性。在一个方面,提供了一种治疗或预防由EphA4介导的疾病或紊乱的方法,包括向需要的受试者施用本文描述的化合物的治疗有效量,包括某些实施例,或结构式(I),(l-A),(II),(III),(IV),(IV-1),(V),(Vl-A),(Vl-B),(VII-1),(VII-2),(VIII-1),或(VIII-2),或其对映体,对映体混合物,两个或更多对映异构体混合物,或其同位素变体;或其药学上可接受的盐,溶剂合物或水合物。
-
Zn- and Cu-Catalyzed Coupling of Tertiary Alkyl Bromides and Oxalates to Forge Challenging C–O, C–S, and C–N Bonds作者:Yuxin Gong、Zhaodong Zhu、Qun Qian、Weiqi Tong、Hegui GongDOI:10.1021/acs.orglett.0c04206日期:2021.2.5We describe here the facile construction of sterically hindered tertiary alkyl ethers and thioethers via the Zn(OTf)2-catalyzed coupling of alcohols/phenols with unactivated tertiary alkyl bromides and the Cu(OTf)2-catalyzed thiolation of unactivated tertiary alkyl oxalates with thiols. The present protocol represents one of the most effective unactivated tertiary C(sp3)–heteroatom bond-forming conditions
-
Fullerene derivatives as dual inhibitors of HIV-1 reverse transcriptase and protease作者:Takumi Yasuno、Tomoyuki Ohe、Hiroki Kataoka、Kosho Hashimoto、Yumiko Ishikawa、Keigo Furukawa、Yasuhiro Tateishi、Toi Kobayashi、Kyoko Takahashi、Shigeo Nakamura、Tadahiko MashinoDOI:10.1016/j.bmcl.2020.127675日期:2021.1In the present study, we newly synthesized three types of novel fullerene derivatives: pyridinium-type derivatives trans-3a and 4a-5b, piperidinium-type derivative 9, and proline-type derivatives 10a-12. Among the assessed compounds, 5a, 10e, 10f, 10i, 11a-d, and 12 were found to inhibit both HIV reverse transcriptase and HIV protease (HIV-PR), with IC50 values in the low micromolar range being observed
-
[EN] SULFONAMIDE COMPOUNDS HAVING TNAP INHIBITORY ACTIVITY<br/>[FR] COMPOSÉS DE SULFONAMIDE AYANT UNE ACTIVITÉ INHIBITRICE DE TNAP申请人:DAIICHI SANKYO CO LTD公开号:WO2018119444A1公开(公告)日:2018-06-28The present invention relates to a compound or a pharmacologically acceptable salt thereof having excellent tissue non-specific alkaline phosphatase inhibitory activity. The present invention provides a compound represented by the formula (I) or a pharmacologically acceptable salt thereof.本发明涉及一种具有优异的组织非特异性碱性磷酸酶抑制活性的化合物或其药理学上可接受的盐。本发明提供一种由式(I)表示的化合物或其药理学上可接受的盐。
-
Chemical Compounds申请人:AstraZeneca AB公开号:US20160376287A1公开(公告)日:2016-12-29Provided are a series of novel pyridine or pyrimidine derivatives which inhibit CDK9 and may be useful for the treatment of hyperproliferative diseases. In particular the compounds are of use in the treatment of proliferative disease such as cancer including hematological malignancies such as acute myeloid leukemia, multiple myeloma, chronic lymphocytic leukemia, diffuse large B cell lymphoma, Burkitt's lymphoma, follicular lymphoma and solid tumors such as breast cancer, lung cancer, neuroblastoma and colon cancer.
表征谱图
-
氢谱1HNMR
-
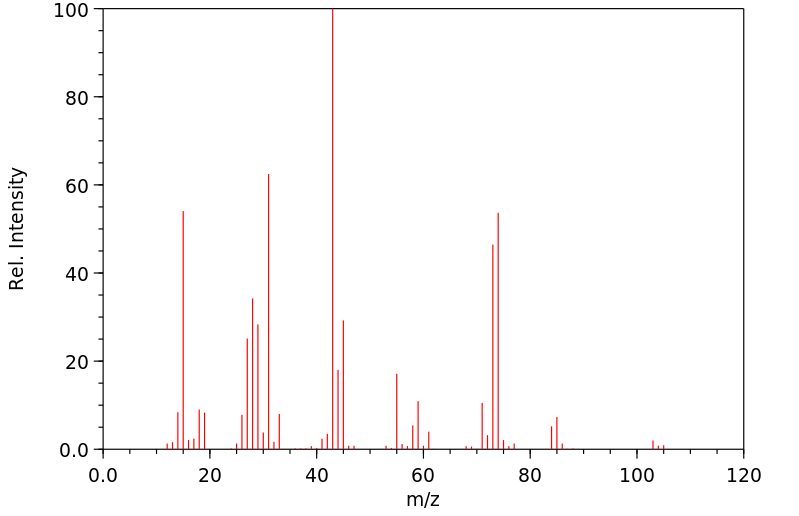
质谱MS
-
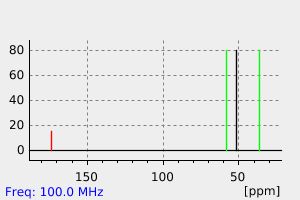
碳谱13CNMR
-
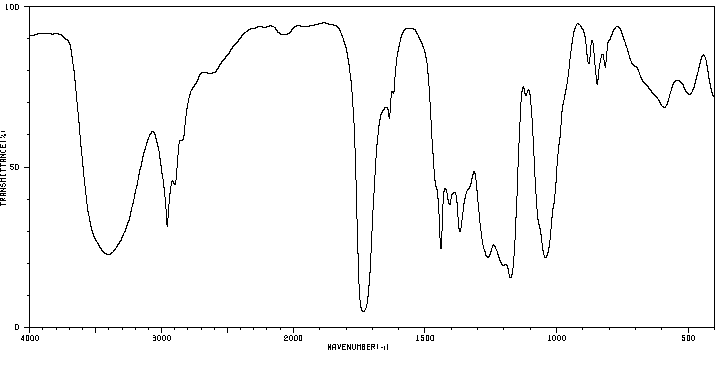
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-2-苯基-3-羟基丙酸
(2S,3R)-2,3-二羟基-3-(2-吡啶基)丙酸乙酯,N-氧化物
麦拉乳酸
阿拉伯碳酸氢二钾
铵;铈(+3)阳离子;(2R,3R)-2,3-二羟基丁烷二酸盐
钡二{8-[3-(2-羟基辛基)-2-环氧乙烷基]辛酸酯}
钠3-脱氧-D-阿拉伯糖-己酮酸酯
钠3-脱氧-D-木糖基-己酮酸酯
钠(3R,5R)-3,5-二羟基-7-[(1S,2S,6R,8S,8aR)-8-羟基-2,6-二甲基-1,2,6,7,8,8A-六氢-1-萘基]庚酸酯
钠(2S)-2-羟基(13C3)丙酸酯
酮酯
酒石酸锂单水合物
酒石酸铬
酒石酸铜(II)一水
酒石酸钾锑
酒石酸钾
酒石酸钠
酒石酸鐵(III)鉀
酒石酸辛酯钠盐
酒石酸羟吡啶
酒石酸氢钾
酒石酸异丙酯
酒石酸二磺基琥珀酰亚胺酯
酒石酸二琥珀酰亚胺酯
酒石酸二戊酯
酒石酸二仲丁酯
酒石酸二丙酯
辛酸,8-氯-6-羟基-,(6R)-
辛伐他汀钾盐
辛伐他汀钠盐
辛伐他汀酸
超支化BIS-MPA聚酯-64-羟基,4代
西托溴铵
表洛伐他汀羟基酸钠盐
葡萄糖酸镍
葡萄糖酸锶
葡萄糖酸锰
葡萄糖酸汞
葡萄糖酸亚铁
莫那可林J酸
苹果酸镁
苹果酸镁
苹果酸铵盐
苹果酸钙
苹果酸氢钠
苹果酸氢钠
苹果酸根
苹果酸二烯丙酯
苹果酸二乙基己酯
苹果酸乙酯(S)-2-羟基丁二酸1-乙酯(苹果酸杂质S)