三甲基(1,2,3,4,5-五甲基环戊二烯基)硅烷 | 87778-95-8
中文名称
三甲基(1,2,3,4,5-五甲基环戊二烯基)硅烷
中文别名
5-(三甲基硅基)-1,2,3,4,5-五甲基-1,3-环戊二烯
英文名称
pentamethylcyclopentadienyltrimethylsilane
英文别名
trimethyl-1,2,3,4,5-pentamethyl-2,4-cyclopentadien-1-yl-silan;SiMe3(C5Me5);5-(Trimethylsilyl)-1,2,3,4,5-pentamethyl-1,3-cyclopentadiene;trimethyl-(1,2,3,4,5-pentamethylcyclopenta-2,4-dien-1-yl)silane
CAS
87778-95-8
化学式
C13H24Si
mdl
MFCD00151193
分子量
208.419
InChiKey
WNTWQEUDFDAMBF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:100-101 °C10 mm Hg(lit.)
-
密度:0.864 g/mL at 25 °C(lit.)
-
闪点:169 °F
-
稳定性/保质期:
按规定使用和贮存的不会分解,应避免接触氧化物、热源及明火。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:14
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.692
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2931900090
-
危险性防范说明:P501,P210,P264,P280,P302+P352,P370+P378,P337+P313,P305+P351+P338,P362+P364,P332+P313,P403+P235
-
危险性描述:H315,H319,H227
-
储存条件:在干燥、阴凉且密闭的环境中,并宜存放在惰性气体中。
SDS
Section 1: Product Identification
Chemical Name: Trimethylsilyl pentamethylcyclopentadiene
CAS Registry Number: 87778-95-8
Formula: [C5(CH3)5]Si(CH3)3
EINECS Number: none
Chemical Family: organosilicon compounds
Synonym: Cyclopentadienyltrimethylsilane
Section 2: Composition and Information on Ingredients
Ingredient CAS Number Percent ACGIH (TWA) OSHA (PEL)
Title Compound 87778-95-8 100% no data no data
Section 3: Hazards Identification
Emergency Overview: May be irritating to skin, eyes, and respiratory tract.
Primary Routes of Exposure: Inhalation, skin, eyes.
Eye Contact: May cause moderate irritation of the eyes.
Skin Contact: May cause slight to mild irritation of the skin.
Inhalation: May cause irritation to the nose, mucous membranes and respiratory tract.
Ingestion: Ingestion may result in the release of a large amount of acetylene gas.
Acute Health Affects: May be irritating to skin, eyes, and respiratory tract.
Chronic Health Affects: No information available on long-term chronic effects.
NTP: No
IARC: No
OSHA: No
SECTION 4: First Aid Measures
Immediately flush the eyes with copious amounts of water for at least 10-15 minutes. A victim may need
Eye Exposure:
assistance in keeping their eye lids open. Get immediate medical attention.
Wash the affected area with water. Remove contaminated clothes if necessary. Seek medical assistance if
Skin Exposure:
irritation persists.
Remove the victim to fresh air. Closely monitor the victim for signs of respiratory problems, such as difficulty
Inhalation:
in breathing, coughing, wheezing, or pain. In such cases seek immediate medical assistance.
Seek medical attention immediately. Keep the victim calm. Give the victim water (only if conscious). Induce
Ingestion:
vomiting only if directed by medical personnel.
SECTION 5: Fire Fighting Measures
Flash Point: no data
Autoignition Temperature: no data
Explosion Limits: no data
Extinguishing Medium: carbon dioxide or dry powder
Fire fighters should be equipped with a NIOSH approved positive pressure self-contained breathing apparatus
Special Fire Fighting Procedures:
and full protective clothing.
Hazardous Combustion and If involved in a fire this material may emit carbon monoxide, carbon dioxide and t
Decomposion Products:
Unusual Fire or Explosion Hazards: Highly flammable. Formation of explosive air/vapor mixtures is possible.
SECTION 6: Accidental Release Measures
Spill and Leak Procedures: Small spills can be absorbed into vermiculite or other suitable adsorbent. Sweep up and dispose of properly.
SECTION 7: Handling and Storage
Handling and Storage: Store the material in a cool, dry place in a tightly sealed container.
SECTION 8: Exposure Controls and Personal Protection
Eye Protection: Always wear approved safety glasses when handling a chemical substance in the laboratory.
Skin Protection: Wear protective clothing and gloves. Consult with glove manufacturer to determine the proper type of glove.
Ventilation: Handle the material in an efficient fume hood.
If ventilation is not available a respirator should be worn. The use of respirators requires a Respirator
Respirator:
Protection Program to be in compliance with 29 CFR 1910.134.
Ventilation: Handle the material in an efficient fume hood.
Additional Protection: No additional protection required.
SECTION 9: Physical and Chemical Properties
Color and Form: light yellow liq.
Molecular Weight: 208.42
Melting Point: no data
Boiling Point: 100°C/10 mm
Vapor Pressure: no data
Specific Gravity: no data
Odor: not determined
Solubility in Water: insoluble
SECTION 10: Stability and Reactivity
Stability: moisture sensitive, (store cold)
Hazardous Polymerization: no hazardous polymerization
Conditions to Avoid: contact with heat and ignition sources.
Incompatibility: Strong oxidizing agents and halogens
Decomposition Products: carbon monoxide, carbon dioxide, silicon dioxide, and organic fumes.
SECTION 11: Toxicological Information
RTECS Data: No information available in the RTECS files.
Carcinogenic Effects: No data available
Mutagenic Effects: No data available
Tetratogenic Effects: No data available
SECTION 12: Ecological Information
Ecological Information: No information available
SECTION 13: Disposal Considerations
Disposal: Dispose of according to federal, state, and local regulations.
SECTION 14: Transportation
Shipping Name (CFR): Flammable liquids, N.O.S.
Hazard Class (CFR): 3
Additional Hazard Class (CFR): NA
Packaging Group (CFR): II
UN ID Number (CFR): UN# 1993
Shipping Name (IATA): Flammable liquid, N.O.S.
Hazard Class (IATA): 3
Additional Hazard Class (IATA): NA
Packaging Group (IATA): II
UN ID Number (IATA): UN# 1993
SECTION 15: Regulatory Information
TSCA: Listed in the TSCA inventory.
SARA (Title 313): Title compound not listed.
Second Ingredient: none
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:Jutzi, Peter; Krato, Bruno; Hursthouse, Mike, Chemische Berichte, 1987, vol. 120, p. 565 - 574摘要:DOI:
-
作为产物:描述:三甲基氯硅烷 、 pentamethylcyclopentadienyllithium 反应 96.0h, 以89%的产率得到三甲基(1,2,3,4,5-五甲基环戊二烯基)硅烷参考文献:名称:合成和动力学Verhalten von Einigen五甲基环戊二烯基硅烷和Germanen摘要:通过使五甲基环戊二烯基锂(pcpLi)与相应的氯硅烷和-锗烷反应,可以合成h 1 -pcp-硅烷和-锗烷I-IX。pcp- chlorogermane VII的亲核取代反应导致h 1 -pcp-germanes X–XIII。从1 H DNMR数据评估了这些化合物中σ重排的活化参数。它们显示出硅或锗上剩余配体对这些动态过程速度的影响很小。DOI:10.1016/0022-328x(83)80055-2
文献信息
-
Synthesis and catalysis of novel mono- and bis(diene) complexes of niobium and x-ray structures of binuclear [Nb(.mu.-Cl)(C5H5)(s-cis-butadiene)]2 and mononuclear Nb(C5H5)(s-cis-2,3-dimethylbutadiene)2作者:Takuji. Okamoto、Hajime. Yasuda、Akira. Nakamura、Yasushi. Kai、Nobuko. Kanehisa、Nobutami. KasaiDOI:10.1021/ja00223a018日期:1988.7Preparation des composes du titre a partir de NbCl 4 (C 5 R 5 ) ou R=H, CH 3 et de butene-2diyl-1,4 magnesium. Les dienes coordinats de ces complexes sont le dimethyl-2,3 butadiene, l'isoprene et le butadiene
-
Sodium–Potassium Alloy for the Reduction of Monoalkyl Aluminum(III) Compounds作者:Mark Schormann、Klaus S. Klimek、Hagen Hatop、Saji P. Varkey、Herbert W. Roesky、Christopher Lehmann、Cord Röpken、Regine Herbst-Irmer、Mathias NoltemeyerDOI:10.1006/jssc.2001.9278日期:2001.12RAlX2 R=Cp* (C5Me5), X=Cl, Br, I (1–3); (BisAlCl2)2 (Bis=(Me3Si)2CH) (5); TrisSi [(Me3Si)3Si], X=Cl, Br, I (6–8); CycTris [(CycMe2Si)(Me3Si)2C], X=Me, F, Cl, Br, I (11–15)} were prepared and characterized by NMR-, IR-, and mass spectroscopy as well as elemental analysis. The single-crystal X-ray structures of Cp*AlBr2, TrisSiAlX2·THF (X=Cl, Br, I), CycTrisAlX2·THF (X=Me, Cl, Br, I), and [CycTrisAl(μ-O(CH2)3CH2)]2的类型的单烷基(III)的化合物[R的Al X 2 - [R =的Cp *(C 5我5),X =氯,溴,I(1-3); (BisAlCl 2)2(Bis =(Me 3 Si)2 CH)(5); TrisSi [(Me 3 Si)3 Si],X = Cl,Br,I(6-8);制备了CycTris [(CycMe 2 Si)(Me 3 Si)2 C],X = Me,F,Cl,Br,I(11-15)},并通过NMR,IR和质谱进行了表征,以及元素分析。Cp的单晶X射线结构* AlBr 2,TrisSiAl X 2 ·THF(X = Cl,Br,I),CycTrisAl X 2 ·THF(X = Me,Cl,Br,I)和[CycTrisAl(μ- O(CH 2)3 CH 2)]报告了2个。使用Na / K合金还原Cp * Al X 2(X = Cl,Br,I)后,分离出单烷基铝(I)化合物(Cp
-
Synthesis, Structural Characterisation and Reactivity of New Dinuclear Monocyclopentadienyl Imidoniobium and ‐tantalum Complexes − X‐ray Crystal Structures of [{Nb(η <sup>5</sup> ‐C <sub>5</sub> H <sub>4</sub> SiMe <sub>3</sub> )Cl <sub>2</sub> } <sub>2</sub> (μ‐1,4‐NC <sub>6</sub> H <sub>4</sub> N)], [{Ta(η <sup>5</sup> ‐C <sub>5</sub> Me <sub>5</sub> )Cl <sub>2</sub> } <sub>2</sub> (μ‐1,4‐NC <sub>6</sub> H <sub>4</sub> N)] and [{Ta(η <sup>5</sup> ‐C <sub>5</sub> Me <sub>5</sub> )(CH <sub>2</sub> SiMe <sub>3</sub> ) <sub>2</sub> } <sub>2</sub> (μ‐1,4‐NC <sub>6</sub> H <sub>4</sub> N)]作者:Antonio Antiñolo、Iván Dorado、Mariano Fajardo、Andrés Garcés、Isabel López‐Solera、Carmen López‐Mardomingo、Marek M. Kubicki、Antonio Otero、Sanjiv PrasharDOI:10.1002/ejic.200300530日期:2004.3R = Me, i = 3 (13); Cp′ = η5-C5H4SiMe3, M = Nb, R = CH2SiMe3, i = 3 (14); Cp′ = η5-C5H4SiMe3, M = Nb, R = CH2Ph, i = 3 (15); Cp′ = η5-C5Me5, M = Ta, R = Me, i = 4 (16); Cp′ = η5-C5Me5, M = Ta, R = CH2SiMe3, i = 4 (17); Cp′ = η5-C5Me5, M = Ta, R = CH2Ph, i = 4 (18)]. The alkylated complexes with other stoichiometries, [M(Cp′)(Me)(X)}2(μ-1,4-NC6H4N)] [Cp = η5-C5H4SiMe3, M = Nb, X = Cl (19); Cp = η5-C5H4SiMe3一系列双核单环戊二烯基亚氨基铌和-钽配合物的制备是通过两种不同的合成路线实现的。二亚胺配合物 [M(Cp')Cl2}2(μ-1,i-NC6H4N)] [Cp' = η5-C5H4SiMe3, M = Nb, i = 4 (1); Cp' = η5-C5H4SiMe3, M = Nb, i = 3 (2); Cp' = η5-C5H4SiMe3, M = Nb, i = 2 (3); Cp' = η5-C5Me5, M = Nb, i = 4 (4); Cp' = η5-C5Me5, M = Nb, i = 3 (5); Cp' = η5-C5H4SiMe3, M = Ta, i = 4 (6); Cp' = η5-C5H4SiMe3, M = Ta, i = 3 (7)] 由一当量的三氯前体 [MCl3}2(μ-1,i-NC6H4N)] (M = Nb, Ta, i = 4, 3 或 2)
-
Preparation of titanium pentamethylcyclopentadienyl trialkyls and crystal structure of tribenzylpentamethylcyclopentadienyltitanium, showing some evidence of a CH2? Ti interaction作者:Miguel Mena、Maria Angela Pellinghelli、Pascual Royo、Ricardo Serrano、Antonio TiripicchioDOI:10.1039/c39860001118日期:——Titanium pentamethylcyclopentadienyl trialkyls (η5-C5Me5)TiR3(R = Me, CH2SiMe3, C6F5, and CH2Ph) are easily obtained by conventional alkylation of the trichloro analogue; the structure of the tribenzyl derivative, determined by X-ray methods, shows some evidence of a CH2⋯ Ti interaction.
-
New Titanatranes: Characterization and Styrene Polymerization Behavior作者:Youngjo Kim、Yonggyu Han、Jeong-Wook Hwang、Myong Woon Kim、Youngkyu DoDOI:10.1021/om0109197日期:2002.3.1they exist in the monomeric form in the solid state and the Ti atom adopts essentially an η5 bonding posture with the Cp‘ ring and a tetradentate bonding mode with the trialkanolatoamine ligand via a transannular interaction from the bridgehead N atom to Ti. All compounds show very high catalytic activity for the syndiospecific polymerization of styrene in the presence of modified methylaluminoxane
表征谱图
-
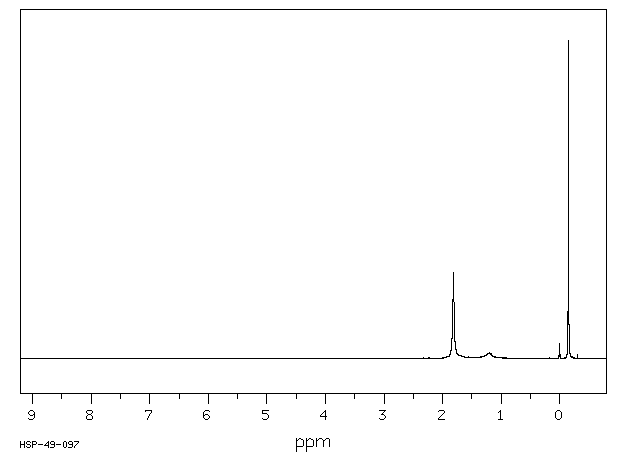
氢谱1HNMR
-
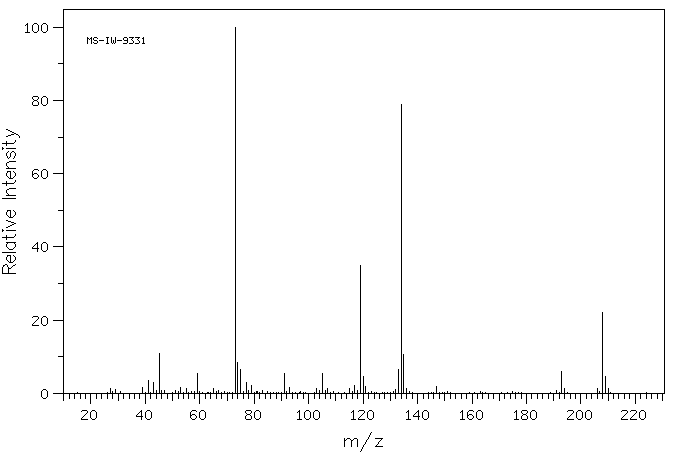
质谱MS
-
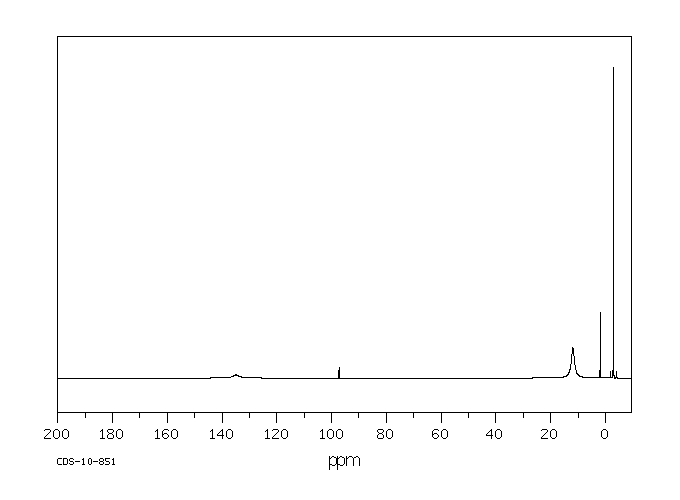
碳谱13CNMR
-
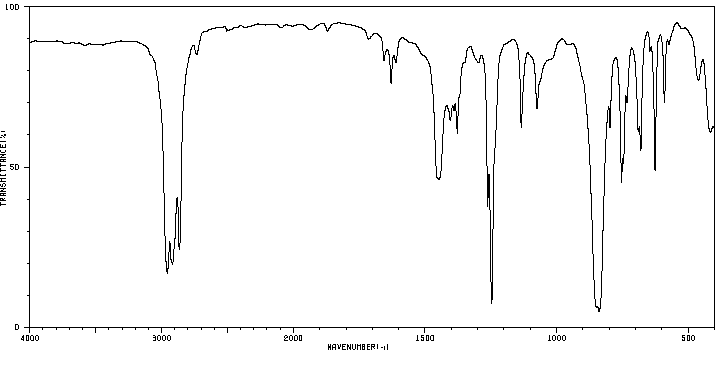
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
镁己烷
锌,二环己基-
锂,3-辛炔基-
锂,(1-苯基乙基)-
铜(I)己基乙炔化物
铜(1+),2-甲基丙烷
铅杂鎓,二乙基甲基-
钠,(1,2,3,4-四甲基-2,4-环戊二烯-1-基)-
钛(4+)四(2,2-二甲基丙烷-1-I去)
邻苯二甲酰基
邻甲基二苯甲酮自由基阳离子
辛烷钠
苄基铜
苄基钠
脱羰秋水仙碱
胂,二(2,2-二甲基丙基)-
纳米碳化钛
红陪酚四甲基醚
红倍酚
秋水仙碱甲硫代磺酸盐
秋水仙碱
碳化锆
碳化铪
碳化铌
碳化铀
碳化钽
碳化钒
碘二氟甲基(1+)
硼化二铬
硫代秋水仙碱
硅烷,二甲基丙基-
硅烷,乙基二甲基-2-丙烯基-
硅烷,乙基二(3-甲基丁基)-
石墨溴化物
甲烷,钼
甲基锡烷
甲基铍氢化物
甲基辛基硅烷
甲基硅烷基阳离子
甲基硅烷
甲基二乙烯基硅烷
甲基丙烯酸7-氧代-4-(苯基偶氮)-1,3,5-环庚三烯-1-基酯
甲基三烯丙基硅烷
甲基三正辛基硅烷
甲基三正己基硅烷
甲基三乙基硅烷
甲基三-N-癸基硅烷
甲基6-肼基-7-氧代-1,3,5-环庚三烯-1-羧酸酯
甲基-三-n-丁基硅烷
甲基(三丙基)硅烷