2-甲基苯并噻吩 | 1195-14-8
中文名称
2-甲基苯并噻吩
中文别名
2-甲基苯并[b]噻吩;2-甲基苯丙[B]噻吩
英文名称
2-Methylbenzothiophene
英文别名
2-methylbenzo[b]thiophene;2-methyl-1-benzothiophene
CAS
1195-14-8
化学式
C9H8S
mdl
MFCD00216250
分子量
148.229
InChiKey
BLZKSRBAQDZAIX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:47-52 °C (lit.)
-
沸点:228.84°C (rough estimate)
-
密度:1.0794 (rough estimate)
-
闪点:113 °C
-
溶解度:溶于甲醇
-
LogP:3.710
-
保留指数:1272.9;1284.6
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.111
-
拓扑面积:28.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2934999090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:保持贮藏器密封,并将其存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
| Name: | 2-Methylbenzo[b]thiophene Material Safety Data Sheet |
| Synonym: | 2-Methylthianaphthene |
| CAS: | 1195-14-8 |
Synonym:2-Methylthianaphthene
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1195-14-8 | 2-Methylbenzo[b]thiophene | 100 | 214-792-2 |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. May be harmful if inhaled.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
Wash mouth out with water. If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1195-14-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 47 - 52 deg C
Autoignition Temperature: Not available.
Flash Point: > 109 deg C (> 228.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
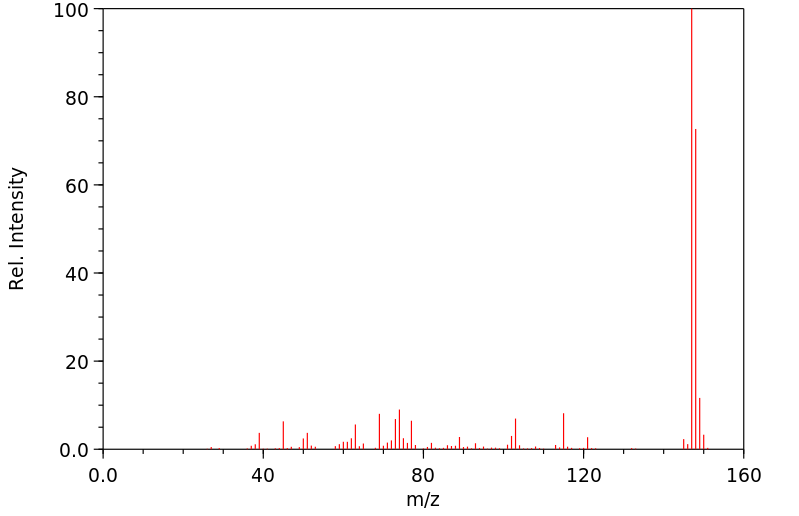
Molecular Formula: C9H8S
Molecular Weight: 148.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1195-14-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Methylbenzo[b]thiophene - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1195-14-8: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1195-14-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1195-14-8 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-苯并噻酚-2-羧醛 2-Benzothiophenecarboxaldehyde 3541-37-5 C9H6OS 162.212 2-(氯甲基)苯并[b]噻吩 2-chloromethylbenzo[b]thiophene 2076-88-2 C9H7ClS 182.674 1-苯并噻吩-2-甲醇 benzothiophene-2-methanol 17890-56-1 C9H8OS 164.228 2-氰基苯并噻吩 2-cyanobenzothiophene 55219-11-9 C9H5NS 159.211 苯并[b]噻吩-2-乙酸 2-(benzo[b]thiophen-2-yl)acetic acid 75894-07-4 C10H8O2S 192.238 苯并噻吩-2-羰酰氯 benzothiophene-2-carboxylic acid chloride 39827-11-7 C9H5ClOS 196.657 苯并噻吩-2-羧酸 Benzo[b]thiophene-2-carboxylic acid 6314-28-9 C9H6O2S 178.211 3-溴-2-甲基-苯并[b]噻吩 3-bromo-2-methylbenzo[b]thiophene 10243-15-9 C9H7BrS 227.125 苯并噻吩 Benzo[b]thiophene 95-15-8 C8H6S 134.202 —— 2-(2-anilinoethyl)benzothiophene 119521-99-2 C16H15NS 253.368 甲基苯并噻吩-2-甲醛 methyl benzo[b]thiophene-2-carboxylate 22913-24-2 C10H8O2S 192.238 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-苯并噻酚-2-羧醛 2-Benzothiophenecarboxaldehyde 3541-37-5 C9H6OS 162.212 1-苯并噻吩-2-甲醇 benzothiophene-2-methanol 17890-56-1 C9H8OS 164.228 苯并[b]噻吩,2-(溴甲基)- 2-bromomethylbenzo[b]thiophene 10133-20-7 C9H7BrS 227.125 苯并[b]噻吩-2-乙腈 benzothiophene-2-acetonitrile 75444-80-3 C10H7NS 173.238 —— (NZ)-N-(1-benzothiophen-2-ylmethylidene)hydroxylamine —— C9H7NOS 177.227 —— 2-(azidomethyl)benzo[b]thiophene 1333393-76-2 C9H7N3S 189.241 苯并[b]噻吩-2-乙酸 2-(benzo[b]thiophen-2-yl)acetic acid 75894-07-4 C10H8O2S 192.238 苯并噻吩-2-羧酸 Benzo[b]thiophene-2-carboxylic acid 6314-28-9 C9H6O2S 178.211 3-溴-2-甲基-苯并[b]噻吩 3-bromo-2-methylbenzo[b]thiophene 10243-15-9 C9H7BrS 227.125 —— 3-iodo-2-methylbenzo[b]thiophene 139625-61-9 C9H7IS 274.125 —— 3-chloro-2-methylbenzothiophene 64845-10-9 C9H7ClS 182.674 —— 3-ethynyl-2-methylbenzo[b]thiophene 1300021-13-9 C11H8S 172.251 2-甲基苯并[b]噻吩-3-羧醛 2-methylbenzo[b]thiophene-3-carbaldehyde 30446-99-2 C10H8OS 176.239 3-氯甲基-2-甲基苯并噻吩 3-chloromethyl-2-methyl-benzo[b]thiophene 16957-90-7 C10H9ClS 196.7 —— 2-methylbenzo[b]thiophene-3-carbonitrile 39812-03-8 C10H7NS 173.238 —— 2-(4-methoxybenzyl)-benzo[b]thiophene 3611-57-2 C16H14OS 254.353 —— (E)-N-(1-benzothiophen-2-ylmethyl)-N,6,6-trimethylhept-2-en-4-yn-1-amine 130219-83-9 C19H23NS 297.464 —— 3-bromo-2-bromomethyl-benzo[b]thiophene 503424-77-9 C9H6Br2S 306.021 —— 2-(4-dimethylaminobenzyl)-benzo[b]thiophene 17347-19-2 C17H17NS 267.395 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:Desulfurization of benzo- and dibenzothiophenes with nickel boride摘要:Nickel boride, prepared from the reduction of nickel chloride hexahydrate with sodium borohydride in methanol-tetrahydrofuran, reduces benzothiophenes to alkylbenzenes and dibenzothiophenes to biphenyls. The reaction is rapid at or below room temperature and does not require protection from the atmosphere. Best results are obtained when the nickel boride is generated in situ in the presence of the sulfur compound. Hydroxyl, carboxyl, ester, and amino groups are unaffected while chloro, bromo, and nitro substituents are also reduced under these conditions. A short-lived intermediate, possibly a nickel hydride species, appears to be required in the desulfurization. Complexation of the substrate to the nickel boride surface, followed by stepwise reduction of the two C-S bonds, occurs. The faster disappearance of dibenzothiophene containing the lighter S-32 isotope compared to that with S-34 (k(S-32)/k(S-34) = 1.005 to 1.006) suggests that C-S bond cleavage is the rate-determining step.DOI:10.1021/jo00033a017
-
作为产物:参考文献:名称:The Vapor Phase Catalytic Synthesis of Thianaphthenes摘要:DOI:10.1021/ja01184a081
-
作为试剂:参考文献:名称:Biphenylsulfonamides and derivatives thereof that modulate the activity of endothelin摘要:本发明提供了二苯基磺酰胺类化合物及其调节或改变内皮素家族肽活性的方法。具体地,提供了双环或三环碳或杂环环二苯基磺酰胺类化合物以及使用这些磺酰胺类化合物通过与受体接触来抑制内皮素肽与内皮素受体结合的方法。本发明还提供了通过给予有效量的这些磺酰胺类化合物或其前药来抑制或增加内皮素活性的方法,用于治疗内皮素介导的疾病。公开号:US06613804B2
文献信息
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
KINASE MODULATORS FOR THE TREATMENT OF CANCER申请人:Synovo GmbH公开号:US20170313661A1公开(公告)日:2017-11-02A method of treating cancer in which a compound that inhibits the expression, production or release of IL-10 by immune cells is combined with a compound that stimulates the production of IL-12 when given in combination with, or in the presence of TNFa. Said method is effective when provided in addition to standard therapies, notably chemotherapy using cytotoxic drugs and other forms of immune therapy including therapeutic vaccines.一种治疗癌症的方法,其中抑制免疫细胞表达、产生或释放IL-10的化合物与在与TNFa联合给予或存在的情况下刺激IL-12产生的化合物结合。所述方法在提供标准疗法的基础上有效,特别是包括使用细胞毒性药物的化疗和其他形式的免疫疗法,包括治疗性疫苗。
-
Cyclopenta[<i>b</i>]annulation of Heteroarenes by Organocatalytic γ′[C(sp<sup>3</sup>)−H] Functionalization of Ynones作者:Moluguri Raghu、Jagdeep Grover、S. S. V. RamasastryDOI:10.1002/chem.201604562日期:2016.12.19to the designed ynones triggers γ′[C(sp3)−H] functionalization, leading to the formation of heteroaryl‐based ortho‐quinodimethane (oQDM) intermediates that undergo carbocyclization to provide cyclopentannulated heteroarenes in good yields and excellent stereoselectivities. Deuterium‐labeling experiments substantiated the proposed reaction mechanism as well as the speculated epimerization.
-
POLYSUBSTITUTED DERIVATIVES OF 2-HETEROARYL-6-PHENYLIMIDAZO[1,2-a]PYRIDINES, AND PREPARATION AND THERAPEUTIC USE THEREOF申请人:DE PERETTI Danielle公开号:US20110065727A1公开(公告)日:2011-03-17Compounds of formula (I): wherein R, R 1 , R 2 , R 3 , R 4 and X are as defined in the disclosure, or an acid addition salt thereof, and the therapeutic use and process of synthesis thereof.式(I)的化合物: 其中R、R1、R2、R3、R4和X如披露中所定义,或其酸盐,以及其治疗用途和合成过程。
-
[EN] CARBOXYL SUBSTITUTED INDOLES FOR USE AS PPAR ALPHA MODULATORS<br/>[FR] INDOLES À SUBSTITUTION CARBOXYLE DESTINÉS À ÊTRE UTILISÉS EN TANT QUE MODULATEURS DU PPAR-ALPHA申请人:SMITHKLINE BEECHAM CORP公开号:WO2009147121A1公开(公告)日:2009-12-10There is provided according to the invention novel compounds of formula (I) or pharmaceutically acceptable salts or solvates thereof wherein one of R1 and R2 is H and the other is COOH. The compounds are useful as PPAR modulators.根据本发明提供了式(I)的新化合物或其药用可接受的盐或溶剂,其中R1和R2中的一个是H,另一个是COOH。这些化合物可用作PPAR调节剂。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
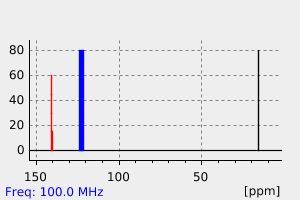
碳谱13CNMR
-
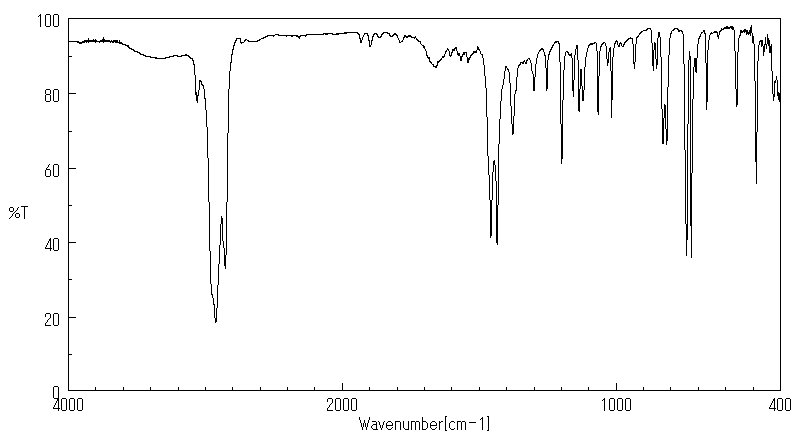
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯