苯并噻吩-2-羧酸肼 | 175135-07-6
中文名称
苯并噻吩-2-羧酸肼
中文别名
苯并[b]噻吩-2-羧酰肼
英文名称
benzo[b]thiophene-2-carbohydrazide
英文别名
benzothiophene-2-carboxylic acid hydrazide;1-Benzothiophene-2-carbohydrazide
CAS
175135-07-6
化学式
C9H8N2OS
mdl
MFCD00052501
分子量
192.241
InChiKey
ZXKPFIRPUUAAPQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:176-178 °C
-
密度:1.367±0.06 g/cm3(Predicted)
-
稳定性/保质期:
避氧化物
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:83.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
安全说明:S26,S37/39
-
储存条件:保存方法:密闭、阴凉、通风干燥处。
SDS
| Name: | benzo[b]thiophene-2-carbohydrazide Material Safety Data Sheet |
| Synonym: | |
| CAS: | 175135-07-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 175135-07-6 | benzo[b]thiophene-2-carbohydrazide | 97 | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Not available.
Skin:
Not available.
Ingestion:
Not available.
Inhalation:
Not available.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
Not available.
Extinguishing Media:
Not available.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Not available.
Section 7 - HANDLING and STORAGE
Handling:
Not available.
Storage:
Not available.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Exposure Limits CAS# 175135-07-6: Personal Protective Equipment Eyes: Not available.
Skin:
Not available.
Clothing:
Not available.
Respirators:
Not available.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white - yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 240 - 242 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H5FN2O
Molecular Weight: 164
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Not available.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 175135-07-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
benzo[b]thiophene-2-carbohydrazide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 175135-07-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 175135-07-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 175135-07-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯并噻吩-2-羧酸 Benzo[b]thiophene-2-carboxylic acid 6314-28-9 C9H6O2S 178.211 甲基苯并噻吩-2-甲醛 methyl benzo[b]thiophene-2-carboxylate 22913-24-2 C10H8O2S 192.238 1-苯并噻吩-2-羧酸乙酯 ethyl benzo[b]thiophene-2-carboxylate 17890-55-0 C11H10O2S 206.265 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-azidocarbonylbenzothiophene 78140-97-3 C9H5N3OS 203.224 —— N-(pyridin-4-yl)benzo[b]thiophene-2-carboxamide —— C14H10N2OS 254.312
反应信息
-
作为反应物:描述:苯并噻吩-2-羧酸肼 在 sodium hydroxide 、 PS-supported BEMP 作用下, 以 1,4-二氧六环 、 乙醇 、 二氯甲烷 为溶剂, 反应 26.17h, 生成 4-{3-Benzo[b]thiophen-2-yl-5-[2-(1H-indol-3-yl)-ethylsulfanyl]-[1,2,4]triazol-4-yl}-butylamine参考文献:名称:3-Thio-1,2,4-triazoles, novel somatostatin sst2/sst5 agonists摘要:Novel 3-thio-1,2,4-triazoles have been obtained via a solution-phase parallel synthesis strategy, affording potent non-peptidic human somatostatin receptor subtypes 2 and 5 agonists. (c) 2005 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2005.05.061
-
作为产物:描述:参考文献:名称:2-(1,3,4-恶二唑-2(3H)-硫酮)-3-氨基-5-芳基噻吩并[2,3-b]吡啶作为DRAK2抑制剂的合成及其构效关系研究摘要:近年来,DAPK相关的凋亡诱导蛋白激酶2(DRAK2)已成为治疗各种自身免疫性疾病和预防器官移植后移植排斥的有希望的靶标。但是,尚未发现用于发现DRAK2新型小分子抑制剂的药物化学优化方案。导致了苯并噻吩类似物的发现专有化合物文库的筛选,其中显示的亲和常数(ķ d 0.25μ)值中号。芯支架的变化时,可以得到一系列5- arylthieno [2,3-的取代模式的b ]与强的结合亲和力的吡啶(ķ d = 0.008μ中号代表最有力的代表)。这些化合物还显示出在功能生化DRAK2酶测定有前途的活性,与IC 50值的0.029μ中号为最有效的同类。最有效的化合物的选择性分析表明,它们在DAPK激酶家族中缺乏选择性。但是,效力较低的类似物之一是DRAK2的选择性配体,可以用作合成选择性有效的DRAK2抑制剂的起点。DOI:10.1002/cmdc.201402234
文献信息
-
신규한 화합물 또는 이의 약학적으로 허용가능한 염, 및 이를 유효성분으로 함유하는 인플루엔자 바이러스 감염으로 인한 질환의 예방 또는 치료용 약학적 조성물申请人:KOREA RESEARCH INSTITUTE OF CHEMICAL TECHNOLOGY 한국화학연구원(319980077651)公开号:KR20150134301A公开(公告)日:2015-12-01본 발명은 신규한 화합물 또는 이의 약학적으로 허용가능한 염, 및 이를 유효성분으로 함유하는 인플루엔자 바이러스 감염으로 인한 질환의 예방 또는 치료용 약학적 조성물에 관한 것이다. 본 발명에 따른 화학식 1로 표시되는 신규한 화합물은 인플루엔자 바이러스에 대한 항바이러스 활성이 현저히 우수할 뿐만 아니라, 인간 세포에 대한 세포독성이 없어 인체에 부작용이 적으므로, 이를 유효성분으로 함유하는 약학적 조성물은 인플루엔자 바이러스 감염에 의해 발병되는 독감, 감기, 인후염, 기관지염, 폐렴, 조류독감, 돼지독감, 염소독감 등의 예방 또는 치료용 유용하게 사용될 수 있다.
-
Substituted indole-2-carboxylic acid benzylidene-hydrazides and analogs as activators of caspases and inducers of apoptosis and the use thereof申请人:——公开号:US20020128292A1公开(公告)日:2002-09-12The present invention is directed to substituted indole-2-carboxylic acid benzylidene-hydrazides and analogs thereof, represented by the general Formula I: 1 wherein X, Ar 1 , R 2 -R 6 and R 12 are defined herein. The present invention also relates to the discovery that compounds having Formula I are activators of caspases and inducers of apoptosis. The compounds of this invention may be used to induce cell death in a variety of clinical conditions in which uncontrolled growth and spread of abnormal cells occurs.
-
Microscale Parallel Synthesis of Acylated Aminotriazoles Enabling the Development of Factor XIIa and Thrombin Inhibitors作者:Simon Platte、Marvin Korff、Lukas Imberg、Ilker Balicioglu、Catharina Erbacher、Jonas M. Will、Constantin G. Daniliuc、Uwe Karst、Dmitrii V. KalininDOI:10.1002/cmdc.202100431日期:2021.12.14approach toward N-acylated aminotriazoles is reported, enabling the compounds’ screening against FXIIa and thrombin. This approach afforded low-nanomolar FXIIa and thrombin inhibitors with no off-targeting of the other tested serine proteases. Selected compounds were shown to be covalent inhibitors of FXIIa and demonstrated anticoagulant properties in vitro, influencing the intrinsic blood coagulation
-
Design, Synthesis, and Pharmacological Evaluation of First‐in‐Class Multitarget <i>N</i> ‐Acylhydrazone Derivatives as Selective HDAC6/8 and PI3Kα Inhibitors作者:Daniel A. Rodrigues、Fabiana S. Guerra、Fernanda S. Sagrillo、Pedro Sena M. Pinheiro、Marina A. Alves、Sreekanth Thota、Lorrane S. Chaves、Carlos M. R. Sant'Anna、Patrícia D. Fernandes、Carlos A. M. FragaDOI:10.1002/cmdc.201900716日期:2020.3.18phosphatidylinositol 3-kinases (PI3Ks) is a very promising approach for cancer treatment. This manuscript describes the design, synthesis, in vitro pharmacological profile, and molecular modeling of a novel class of N-acylhydrazone (NAH) derivatives that act as HDAC6/8 and PI3Kα dual inhibitors. The surprising selectivity for PI3Kα may be related to differences in the conformation in the active site
-
PYRAZOLE COMPOUNDS AND USE THEREOF申请人:Takagi Masaki公开号:US20090036450A1公开(公告)日:2009-02-05The pyrazole compound of the present invention is represented by the following general formula (I). The pyrazole compound of the present invention or a salt thereof or a solvate thereof potently inhibits liver glycogen phosphorylase, and, therefore, is useful as a therapeutic or prophylactic agent for diabetes. wherein each symbol denotes as described in the specifications.
表征谱图
-
氢谱1HNMR
-
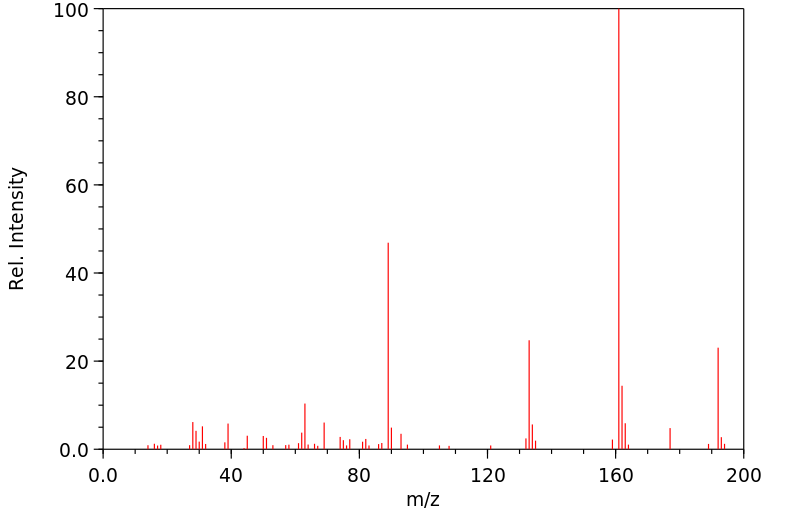
质谱MS
-
碳谱13CNMR
-
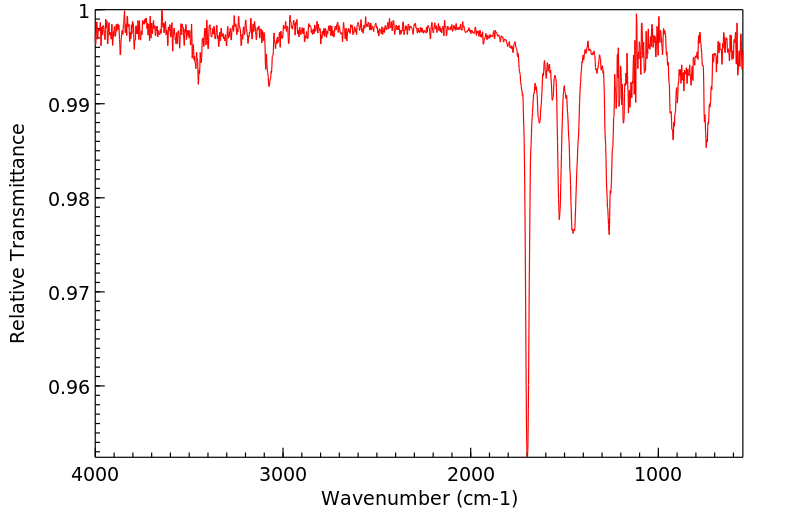
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐留通钠
齐留通相关物质A
齐留通亚砜
齐留通-d4
齐留通
雷洛昔芬杂质
邻联甲苯胺砜
试剂4,8-Bis(3,5-dioctyl-2-thienyl)-2,6-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[1,2-b:4,5-b']dithiophene
试剂1,1'-[4,8-Bis[4-(2-ethylhexyl)-3,5-difluorophenyl]benzo[1,2-b:4,5-b']dithiophene-2,6-diyl]bis[1,1,1-trimethylstannane]
苯并噻吩-7-醇
苯并噻吩-4-硼酸频哪醇酯
苯并噻吩-3-羧酸甲酯
苯并噻吩-3-硼酸
苯并噻吩-2-羰酰氯
苯并噻吩-2-羧酸肼
苯并噻吩-2-羧酸
苯并噻吩-2-硼酸
苯并噻吩-2-氨基甲酸叔丁酯
苯并噻吩
苯并[c]噻吩
苯并[b]噻吩-7-胺
苯并[b]噻吩-7-羧酸乙酯
苯并[b]噻吩-7-甲醛
苯并[b]噻吩-7-甲腈
苯并[b]噻吩-6-醇
苯并[b]噻吩-6-胺
苯并[b]噻吩-6-羧酸乙酯
苯并[b]噻吩-6-羧酸
苯并[b]噻吩-6-甲腈
苯并[b]噻吩-5-甲腈,2-甲酰基-
苯并[b]噻吩-5-甲磺酰氯
苯并[b]噻吩-4-羧酸甲酯
苯并[b]噻吩-4-羧酸
苯并[b]噻吩-4-甲醛
苯并[b]噻吩-4-甲腈
苯并[b]噻吩-4-基甲醇
苯并[b]噻吩-3-胺盐酸盐
苯并[b]噻吩-3-胺
苯并[b]噻吩-3-羧酸-(2-二烯丙基氨基乙酯)
苯并[b]噻吩-3-硼酸频哪酯
苯并[b]噻吩-3-甲醛肟
苯并[b]噻吩-3-甲酰胺
苯并[b]噻吩-3-基乙酸酯
苯并[b]噻吩-3-乙酸
苯并[b]噻吩-3-乙酰氯
苯并[b]噻吩-3-乙腈
苯并[b]噻吩-2-胺盐酸盐
苯并[b]噻吩-2-羧酸6-氨基-3-氯-甲酯
苯并[b]噻吩-2-羧酸,5-氯-3-(1-甲基乙氧基)-
苯并[b]噻吩-2-羧酸,3-羟基-5-甲氧基-,甲基酯