4-羟基-5-甲基-2-己酮 | 38836-21-4
中文名称
4-羟基-5-甲基-2-己酮
中文别名
——
英文名称
4-hydroxy-5-methyl-hexan-2-one
英文别名
4-hydroxy-5-methyl-2-hexanone;5-methyl-4-hydroxy-2-hexanone;4-hydroxy-5-methylhexan-2-one
CAS
38836-21-4
化学式
C7H14O2
mdl
——
分子量
130.187
InChiKey
BXQODTBLKZARFD-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:90 °C(Press: 16 Torr)
-
密度:0.9432 g/cm3(Temp: 22 °C)
-
LogP:0.478 (est)
-
保留指数:954
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:9
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二丙酮醇 4-Hydroxy-4-methyl-2-pentanone 123-42-2 C6H12O2 116.16
反应信息
-
作为反应物:描述:参考文献:名称:The E2C mechanism in elimination reactions. 8. Interaction of conjugating substituents with E2C- and E2H-like transition states摘要:DOI:10.1021/jo00881a030
-
作为产物:参考文献:名称:硫修饰的双功能水滑石的合成及其反相气相色谱法研究其表面特性†摘要:在这项研究中,制备了各种硫改性的水滑石催化剂,并研究了煅烧温度对其酸碱性质的影响。使用X射线粉末衍射,扫描电子显微镜,N 2研究了催化剂的结构表征物理吸附,元素分析和傅立叶变换红外光谱。结构表征表明保留了所有催化剂的层结构,但是比表面积增大了。通过计算热力学参数,包括分散的表面自由能,吸附自由能,吸附焓和酸碱相互作用常数,进行了反相气相色谱定量测定催化剂的酸碱性质。结果表明,随着煅烧温度的升高,酸性和碱性位的强度和含量均增加。此外,选择了几种典型的醛醇缩合反应来研究已开发的催化剂的催化活性。DOI:10.1039/c5cy00765h
文献信息
-
Improved Conditions for the Proline-Catalyzed Aldol Reaction of Acetone with Aliphatic Aldehydes作者:Benjamin List、Alberto Martínez、Kristina Zumbansen、Arno Döhring、Manuel van GemmerenDOI:10.1055/s-0033-1340919日期:——The proline-catalyzed asymmetric aldol reaction between aliphatic aldehydes and acetone has, to date, remained underdeveloped. Challenges in controlling this reaction include avoiding undesired side reactions such as aldol condensation and self-aldolization. In recent years we have developed optimized conditions, which enable high yields and good to excellent enantioselectivities, and which are presented
-
Processes for preparing beta-hydroxy-ketones and alpha,beta-unsaturated ketones申请人:Barnicki Donald Scott公开号:US20050004401A1公开(公告)日:2005-01-06Processes for producing β-hydroxy-ketones and α,β-unsaturated ketones are disclosed which comprise the crossed condensation of an aldehyde with a ketone in the presence of a hydroxide or alkoxide of alkali metal or an alkaline earth metal as catalyst. The products of the process, β-hydroxy-ketones and α,β-unsaturated ketones, are useful for the preparation of many commercially important products in the chemical process industries including solvents, drug intermediates, flavors and fragrances, other specialty chemical intermediates.
-
Direct organocatalytic aldol reactions in buffered aqueous media作者:Armando Córdova、Wolfgang Notz、Carlos F. Barbas IIIDOI:10.1039/b207664k日期:——Organocatalytic cross-aldol reactions catalyzed by cyclic secondary amines in aqueous media provide a direct route to a variety of aldols including carbohydrate derivatives and may warrant consideration as a prebiotic route to sugars.
-
Studies on the Origin of 1,5-anti Induction in Boron-Mediated Aldol Reactions作者:Bridget L. Stocker、Paul Teesdale-Spittle、John O. HobergDOI:10.1002/ejoc.200300592日期:2004.1A model for the origin of selectivity in boron-mediated 1,5-anti-aldols is presented. This model involves π-stacking between the boron enolate and a remote aromatic ring. A short, facile method for the synthesis of the C-12 to C-22 segment of peloruside A and its 1,5-anti-aldol coupling using the proposed model is also presented. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2004)
-
Au(I)-Catalyzed Cyclization of<i>tert</i>-Butyl Carbonates Derived from Homopropargyl Alcohols: A Catalytic Alternative to Cyclic Enol Carbonates作者:Seunghoon Shin、Ji-Eun KangDOI:10.1055/s-2006-933110日期:——Au(I)-complexes catalyze cyclization of tert-butyl carbonates derived from a variety of homopropargyl alcohols. This procedure offers a catalytic alternative to stoichiometric Lewis acids for the preparation of a range of enol carbonates.
表征谱图
-
氢谱1HNMR
-
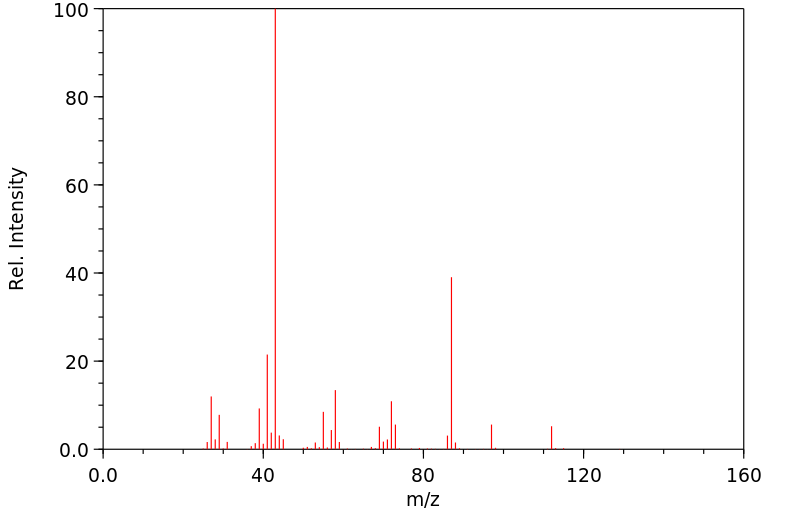
质谱MS
-
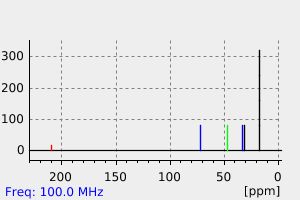
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷