顺式-3-癸烯 | 19398-86-8
中文名称
顺式-3-癸烯
中文别名
——
英文名称
cis-3-decene
英文别名
cis-3-Decen;(Z)-3-decene;(Z)-3-Decen;(Z)-dec-3-ene
CAS
19398-86-8
化学式
C10H20
mdl
——
分子量
140.269
InChiKey
GVRWIAHBVAYKIZ-ALCCZGGFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-56.93°C (estimate)
-
沸点:166.82°C (estimate)
-
密度:0.7631 (estimate)
-
LogP:5.551 (est)
-
保留指数:995;995;995;996;997;986;993
计算性质
-
辛醇/水分配系数(LogP):4.7
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 正癸烯 1-Decene 872-05-9 C10H20 140.269
反应信息
-
作为反应物:参考文献:名称:An efficient didehydroxylation method for the biomass-derived polyols glycerol and erythritol. Mechanistic studies of a formic acid-mediated deoxygenation摘要:发现了一种高效的1,2-脱氧方法,涉及一种意外的机理,适用于简单二醇和生物质衍生多元醇(甘油和赤藓糖醇),能够将1,2-二羟基转化为碳-碳双键。DOI:10.1039/b907746d
-
作为产物:描述:参考文献:名称:合成砜类(XXIV)合成醇类合成乙烯砜。摘要:现在可用的E或Z乙烯基砜可以用连二亚硫酸钠立体定向还原为相应的烯烃。DOI:10.1016/s0040-4039(00)87587-5
文献信息
-
A Selective Ru-Catalyzed Semireduction of Alkynes to Z Olefins under Transfer-Hydrogenation Conditions作者:Christian Belger、N. Matthias Neisius、Bernd PlietkerDOI:10.1002/chem.201001143日期:2010.10.25By using a readily available, air‐ and moisture‐stable dihydrido–Ru complex, a variety of Z olefins are accessible under transfer‐hydrogenation conditions with formic acid as the hydrogen source in excellent yields and Z/E selectivities.
-
Anti-Markovnikov Hydroheteroarylation of Unactivated Alkenes with Indoles, Pyrroles, Benzofurans, and Furans Catalyzed by a Nickel–<i>N</i>-Heterocyclic Carbene System作者:York Schramm、Makoto Takeuchi、Kazuhiko Semba、Yoshiaki Nakao、John F. HartwigDOI:10.1021/jacs.5b08039日期:2015.9.30benzofurans, and furans, to unactivated terminal and internal alkenes. The reaction is catalyzed by a combination of Ni(COD)2 and a sterically hindered, electron-rich N-heterocyclic carbene ligand or its analogous Ni(NHC)(arene) complex. The reaction is highly selective for anti-Markovnikov addition to α-olefins, as well as for the formation of linear alkylheteroarenes from internal alkenes. The reaction occurs
-
[EN] TRANSITION METAL COMPLEXES<br/>[FR] TRANSITION METAL COMPLEXES申请人:SHELL INT RESEARCH公开号:WO2005090371A1公开(公告)日:2005-09-29A transition metal complex which is a bis-arylimine pyridine MXn complex, comprising a bis-arylimine pyridine ligand having the formula (I), wherein R1-R5, R7-R9, R12 and R14 are each, independently, hydrogen, optionally substituted hydrocarbyl, an inert functional group, or any two of R1-R3 and R7-R9 vicinal to one another taken together may form a ring, and R6 is hydrogen, optionally substituted hydrocarbyl, an inert functional group, or taken together with R7 or R4 to form a ring, R10 is hydrogen, optionally substituted hydrocarbyl, an inert functional group, or taken together with R9 or R4 to form a ring, R11 is hydrogen, optionally substituted hydrocarbyl, an inert functional group, or taken together with R12 or R5 to form a ring, R15 is hydrogen, optionally substituted hydrocarbyl, an inert functional group, or taken together with R14 or R5 to form a ring, provided that R13 and at least one of R12 and R14 are independently selected from optionally substituted C1-C30 alkyl, optionally substituted C4-C30 alkyloxy, halogen and optionally substituted C5-C20 aryl, or R13 taken together with R12 or R14 form a ring, or R12 taken together with R11 form a ring and R14 taken together with R15 form a ring, and provided that at least one of R12, R13 and R14 is optionally substituted C4-C30 alkyloxy; M is a transition metal atom in particular selected from Ti, V, Cr, Mn, Fe, Co, Ni, Pd, Rh, Ru, Mo, Nb, Zr, Hf, Ta, W, Re, Os, Ir or Pt; n matches the formal oxidation state of the transition metal atom M; and X is halide, optionally substituted hydrocarbyl, alkoxide, amide, or hydride. The transition metal complexes of the present invention, their complexes with non-coordinating anions and catalyst systems containing such complexes have good solubility in non-polar media and chemically inert non-polar solvents especially aromatic hydrocarbon solvents. The catalyst systems can be used for a wide range of (co)oligomerization, polymerization and dimerization reactions.一种过渡金属配合物,为双芳胺吡啶MXn配合物,包括具有化学式(I)的双芳胺吡啶配体,其中R1-R5、R7-R9、R12和R14分别为独立的氢、可选择取代的烃基、惰性官能团,或R1-R3和R7-R9中的任意两个邻位取代基可以形成环,R6为氢、可选择取代的烃基、惰性官能团,或与R7或R4一起形成环,R10为氢、可选择取代的烃基、惰性官能团,或与R9或R4一起形成环,R11为氢、可选择取代的烃基、惰性官能团,或与R12或R5一起形成环,R15为氢、可选择取代的烃基、惰性官能团,或与R14或R5一起形成环,其中R13和至少一个R12和R14独立地选自可选择取代的C1-C30烷基、可选择取代的C4-C30烷氧基、卤素和可选择取代的C5-C20芳基,或R13与R12或R14一起形成环,或R12与R11一起形成环,R14与R15一起形成环,且至少一个R12、R13和R14为可选择取代的C4-C30烷氧基;M为过渡金属原子,特别选自Ti、V、Cr、Mn、Fe、Co、Ni、Pd、Rh、Ru、Mo、Nb、Zr、Hf、Ta、W、Re、Os、Ir或Pt;n与过渡金属原子M的形式氧化态相匹配;X为卤素、可选择取代的烃基、烷氧基、酰胺或氢化物。本发明的过渡金属配合物及其与非配位阴离子的复合物和含有此类配合物的催化系统在非极性介质和化学惰性非极性溶剂中具有良好的溶解性,特别是芳香烃类溶剂。该催化系统可用于广泛的(共)寡聚化、聚合和二聚化反应。
-
Selective Semihydrogenation of Alkynes on Shape-Controlled Palladium Nanocrystals作者:Jooyoung Chung、Chanhoi Kim、Hansaem Jeong、Taekyung Yu、Do Huy Binh、Jyongsik Jang、Jaichan Lee、B. Moon Kim、Byungkwon LimDOI:10.1002/asia.201201166日期:2013.5selective semihydrogenation of alkynes to alkenes on shape‐controlled palladium (Pd) nanocrystals was performed. Pd nanocrystals with a cubic shape and thus exposed 100} facets were synthesized in an aqueous solution through the reduction of Na2PdCl4 with L‐ascorbic acid in the presence of bromide ions. The Pd nanocubes were tested as catalysts for the semihydrogenation of various alkynes such as 5‐decyne
-
The Reaction of Alkenylboranes with Palladium Acetate. Stereoselective Synthesis of Olefinic Derivatives作者:Hidetaka YatagaiDOI:10.1246/bcsj.53.1670日期:1980.6The reactions of alkenylboranes with palladium acetate were investigated. Alkenyldialkylboranes, derived from terminal alkynes, underwent intramolecular migration reaction in the presence of an equimolar amount of palladium acetate and triethylamine to give (E)-olefins. On the other hand, under the same conditions as above or even in the presence of catalytic amounts of palladium acetate, alkenyldialkylboranes
表征谱图
-
氢谱1HNMR
-
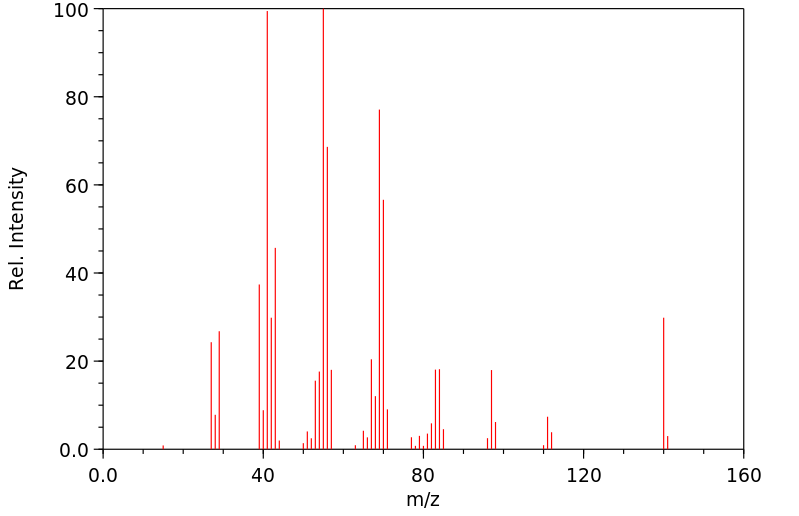
质谱MS
-
碳谱13CNMR
-
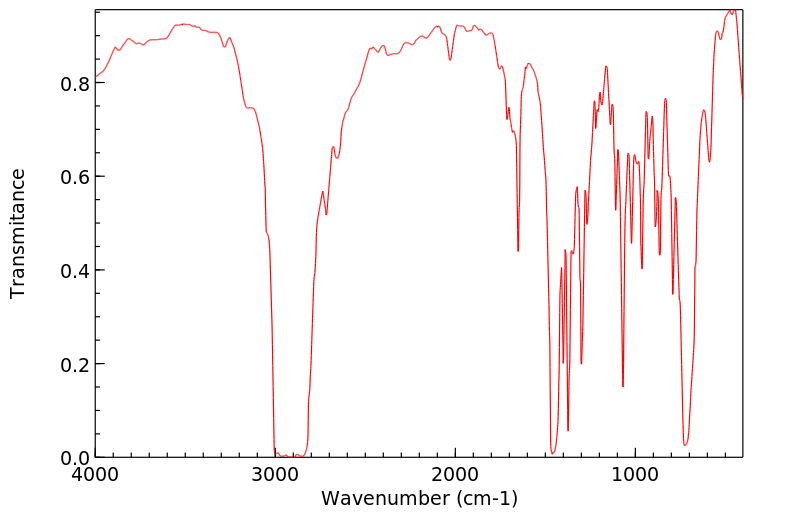
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-