2,5-二溴-3,6-二羟对苯醌 | 4370-59-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:270 °C
-
沸点:328.5±42.0 °C(Predicted)
-
密度:2.53 g/cm3
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
稳定性/保质期:
常温常压下稳定,熔点为270℃。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:74.6
-
氢给体数:2
-
氢受体数:4
安全信息
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Bromanilic Acid
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Bromanilic Acid
Percent: >98.0%(T)
CAS Number: 4370-59-6
Synonyms: 2,5-Dibromo-3,6-dihydroxy-p-quinone
C6H2Br2O4
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Bromanilic Acid
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Light-sensitive
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Yellow - Yellow red
Colour:
Bromanilic Acid
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Odour: No data available
pH: No data available
Melting point/freezing point:270°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Bromanilic Acid
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,5-diacetoxy-3,6-dibromo-[1,4]benzoquinone —— C10H6Br2O6 381.962
反应信息
-
作为反应物:描述:参考文献:名称:全合成合成鞘磷脂G的结构修饰及其对TNF-α产生的抑制活性摘要:本文描述了鞘磷脂G的全合成,从而将提出的结构1修改为2。关键步骤涉及Suzuki-Miyaura双偶联和酯化反应。通过类似的策略,还制备了ganbajunins D和E(3和4)。化合物2强烈抑制大鼠嗜碱性白血病(RBL-2H3)细胞中TNF(肿瘤坏死因子)-α的产生:IC 50 = 3.5 nM,而1和其区域异构体15的混合物则没有这种活性。DOI:10.1021/jo900638b
-
作为产物:描述:参考文献:名称:四氧戊烯桥联的稀土配合物:自由基桥联的双核Dy单分子磁体† •摘要:中性tetraoxolene桥接的通式的双核稀土配合物的两个家庭[((HBpz 3)2 RE)2(μ-tetraoxolene)](RE = Y和Dy; HBpz 3 - =氢三(吡唑基)硼酸盐; tetraoxolene = fluoranilate (fa 2-;1-RE)或溴甲酸酯(ba 2-;2-RE)已经合成并表征。在每种情况下,桥接tetraoxolene配体是在抗磁双阴离子形式,并且每个稀土金属中心具有两个HBpz 3 -配体完成协调。可溶性2-RE的电化学研究家族揭示了基于四氧戊烯的可逆单电子还原。用钴茂金属进行批量化学还原可得到1e还原的类似物的钴ce(CoCp +)盐:[CoCp] [(((HBpz 3)2 RE)2(μ-ba˙)](3-RE),其中包含自由基三阴离子溴酸盐桥联配体的形式。对2-Dy的交流(ac)磁化率研究表明,仅在施加磁场的情况下,磁弛豫缓慢,但是还原DOI:10.1039/c9dt01320b
文献信息
-
Utilizing proton transfer to produce molecular salts in bromanilic acid substituted-pyridine molecular complexes – predictable synthons?作者:Lynne H. Thomas、Martin S. Adam、Andrew O'Neill、Chick C. WilsonDOI:10.1107/s0108270113029533日期:2013.11.15
Controlled introduction of proton transfer into the design of a series of molecular complexes is described, delivering the systematic production of ionic molecular complexes (molecular salts). The controlled production of molecular salts has relevance as a potential strategy in the design of pharmaceutical materials. In nine molecular complexes consisting of bromanilic acid with the N-heterocyclic compounds 2-, 3- and 4-picoline [bis(2/3/4-methylpyridinium) 2,5-dibromo-3,6-dioxocyclohexa-1,4-diene-1,4-diolate, 2C6H8N+·C6Br2O42−], 2,3-, 2,4-, 2,5- and 3,5-lutidine [2,3/2,4/2,5/3,5-dimethylpyridinium 2,5-dibromo-4-hydroxy-3,6-dioxocyclohexa-1,4-dien-1-olate, C7H10N+·C6HBr2O4−], and 3-bromo-4-methylpyridine [3-bromo-4-methylpyridinium 2,5-dibromo-4-hydroxy-3,6-dioxocyclohexa-1,4-dien-1-olate, C6H7BrN+·C6HBr2O4−] and 2-bromo-3-methylpyridine [2-bromo-3-methylpyridine–2,5-dibromo-3,6-dihydroxycyclohexa-2,5-diene-1,4-dione (1/1), C6H6BrN·C6H2Br2O4], proton transfer occurs readily between the bromanilic acid molecule and the N heteroatom of the pyridine ring, in all cases producing a charge-assisted bifurcated N—H...O hydrogen bond. This reinforces the value of this motif as a design tool in the crystal engineering of such complexes. The protonation state (and stoichiometry) significantly affect the supramolecular synthons obtained, but 1:2 stoichiometries reliably give rise to PBP synthons and 1:1 stoichiometries to PBBP synthons (where P indicates a methylpyridine co-molecule and B a bromanilic acid molecule). The influence of halogen interactions on the wider crystal packing is also discussed, with C—H...Br and Br...O interactions the most prevalent; only one Br...Br interaction is found.
介绍了在一系列分子络合物的设计中可控地引入质子传递,从而系统地生产出离子分子络合物(分子盐)。分子盐的受控生产是设计药物材料的一种潜在策略。在由溴苯胺与 N-杂环化合物 2-、3-和 4-甲基吡啶[bis(2/3/4-methylpyridinium) 2,5-dibromo-3,6-dioxocyclohexa-1,4-diene-1,4-diolate, 2C6H8N+-C6Br2O42-]组成的九种分子络合物中、2,3-、2,4-、2,5-和 3,5-lutidine [2,3/2,4/2,5/3,5-dimethylpyridinium 2,5-dibromo-4-hydroxy-3,6-dioxocyclohexa-1,4-dien-1-olate, C7H10N+-C6HBr2O4-], 和 3-bromo-4-methylpyridine [3-bromo-4-methylpyridinium 2、5-二溴-4-羟基-3,6-二氧代环己-1,4-二烯-1-醇,C6H7BrN+-C6HBr2O4-]和 2-溴-3-甲基吡啶[2-溴-3-甲基吡啶-2,5-二溴-3,6-二羟基环己-2,5-二烯-1,4-二酮 (1/1)、C6H6BrN-C6H2Br2O4],溴苯胺分子与吡啶环的 N 杂原子之间很容易发生质子转移,在所有情况下都会产生电荷辅助的 N-H..... O 氢键。.O氢键。这加强了该图案作为此类复合物晶体工程设计工具的价值。质子化状态(和化学计量)对所获得的超分子合成物有很大影响,但 1:2 的化学计量能可靠地产生 PBP 合成物,1:1 的化学计量能产生 PBBP 合成物(其中 P 表示甲基吡啶共分子,B 表示溴苯胺分子)。此外,还讨论了卤素相互作用对更广泛晶体结构的影响,其中 C-H...Br 和 Br...O 相互作用最为普遍;只发现了一种 Br...Br 相互作用。 -
Solvent-modulated structures in anilato-based 2D coordination polymers作者:Samia Benmansour、Irene Pérez-Herráez、Gustavo López-Martínez、Carlos J. Gómez GarcíaDOI:10.1016/j.poly.2017.06.052日期:2017.10O, DMSO (dimethylsulfoxide) and DMF (dimethylformamide). Here we report the synthesis, crystal structure and magnetic characterization of compounds [Er 2 (C 6 O 4 Br 2 ) 3 (H 2 O) 6 ]·12H 2 O ( 1 ), [Er 2 (C 6 O 4 Br 2 ) 3 (DMSO) 4 ]·2DMSO·2H 2 O ( 2 ) and [Er 2 (C 6 O 4 Br 2 ) 3 (DMF) 6 ] ( 3 ). We show the key role played by the shape and size of the three solvents in (i) the coordination number摘要这项工作强调了溶剂在一系列二维化合物[Ln 2(C 6 O 4 Br 2)3·(Solvent)n]·G(G = Guest)的结构中的关键作用。这项研究基于这些溶剂与镧系元素的亲和力和能力以及它们作为溶剂化分子的可能性。为此,在此我们重点研究Er(III)离子和溴化桥联配体([C 6 O 4 Br 2] 2− = 3,6-二溴-2,5-二羟基-1,4-苯醌的二价阴离子),我们使用三种溶剂:H 2 O,DMSO(二甲基亚砜)和DMF(二甲基甲酰胺)。在这里我们报告化合物[Er 2(C 6 O 4 Br 2)3(H 2 O)6]·12H 2 O(1),[Er 2(C 6 O 4 Br 2) )3(DMSO)4]·2DMSO·2H 2 O(2)和[Er 2(C 6 O 4 Br 2)3(DMF)6](3)。我们显示了三种溶剂的形状和大小在(i)金属离子的配位数和几何形状,(ii)这些(6,3)-2D晶格的六方腔畸变,((
-
Selective Encapsulation and Separation of Dihalobenzene Isomers with Discrete Heterometallic Macrocages作者:Hai‐Ning Zhang、Ye Lu、Wen‐Xi Gao、Yue‐Jian Lin、Guo‐Xin JinDOI:10.1002/chem.201805383日期:2018.12.17feature half‐sandwich rhodium(III) fragments at the vertices. Remarkably, a stable cage‐like heteropolymetallic complex possessing eight rhodium(III) and two silver(I) metal ions (3) has been obtained following a multistep procedure. The RhIII/AgI mixed macrocage enables the separation of dihalogenated benzene derivatives with high selectivity. Furthermore, a detailed X‐ray crystallographic study confirmed
-
2D and 3D Anilato-Based Heterometallic M(I)M(III) Lattices: The Missing Link作者:Samia Benmansour、Cristina Vallés-García、Patricia Gómez-Claramunt、Guillermo Mínguez Espallargas、Carlos J. Gómez-GarcíaDOI:10.1021/acs.inorgchem.5b00451日期:2015.6.1The similar bis-bidentate coordination mode of oxalato and anilato-based ligands is exploited here to create the first examples of 2D and 3D heterometallic lattices based on anilato ligands combining M(I) and a M(III) ions, phases already observed with oxalato but unknown with anilato-type ligands. These lattices are prepared with alkaline metal ions and magnetic chiral tris(anilato)metalate molecular本文利用草酸根和基于茴香酸的配体的类似双双配位模式,基于结合M(I)和M(III)离子的茴香酸配体创建了2D和3D杂金属晶格的第一个实例,这些相已用草酸根观察到。但对芳香型配体未知。这些晶格是用碱金属离子和磁性手性三(芳基)金属酸盐分子构建基块制备的:[M III(C 6 O 4 X 2)3 ] 3- [M III = Fe和Cr; X = Cl和Br;( C 6 O 4 X 2)2–= 2,5-二羟基-1,4-苯醌的3,6-二取代衍生物的二价阴离子,H 4 C 6 O 4)。新化合物包括两个非常相似的2D晶格,分别是(PBu 3 Me)2 [NaCr(C 6 O 4 Br 2)3 ](1)和(PPh 3 Et)2 [KFe(C 6 O 4 Cl 2)3 ](dmf)2(2),都呈现六角形[M I M III(C 6 O 4 X2)3 ] 2 –蜂窝层,在它们之间插入(PBu 3 Me)+
-
Effects of ligand substituents on the single-molecule magnetic behavior of quinonoid-bridged dicobalt compounds作者:Xiao-Quan Zhu、Wen-Hai Cao、Shao-Dong Su、Xin-Tao Wu、Tian-Lu ShengDOI:10.1039/d0dt00033g日期:——quinonoid-bridged dicobalt compounds [(N4Co)2LX](ClO4)2 (1-4) (X = H, Cl, Br and OMe; N4 = 1,4,7,10-tetrabenzyl-1,4,7,10-tetraazacyclododecane) are synthesized and well characterized. Single crystal X-ray diffraction analyses reveal that the coordination geometry of one side Co in compounds 1-4 changes from a triangular prism to distorted octahedron with a change in the bridged-ligand substituent. Magnetic measurements
表征谱图
-
氢谱1HNMR
-
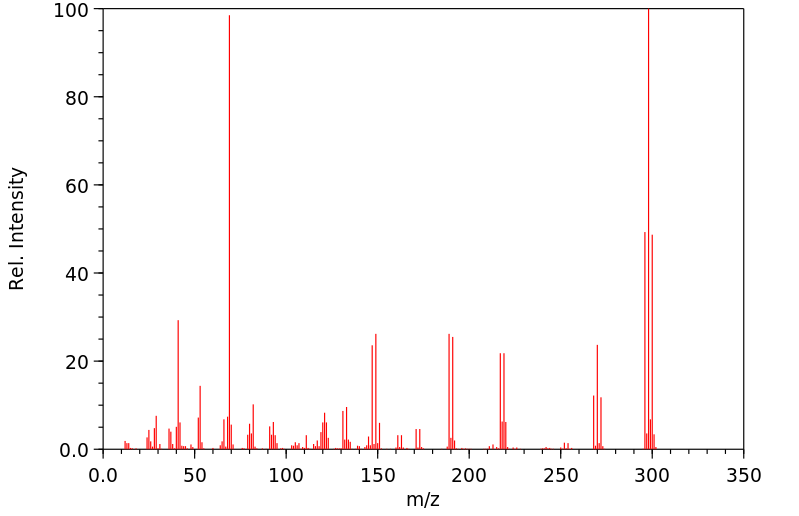
质谱MS
-
碳谱13CNMR
-
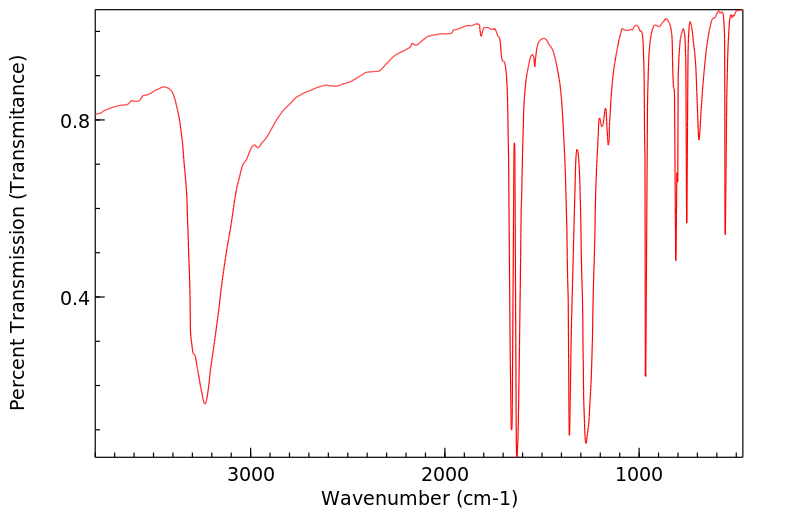
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息