3-甲基-1,3-苯并噁唑-2(3H)-硫酮 | 13673-63-7
中文名称
3-甲基-1,3-苯并噁唑-2(3H)-硫酮
中文别名
3-甲基-2(3H)-苯丙唑硫酮;3-甲基-2(3H)-苯丙噁唑硫酮
英文名称
3-methylbenzoxazole-2-thione
英文别名
3-Methyl-benzoxazolin-2-thion;3-Methyl-2-benzoxazolinthion;3-methylbenzoxazoline-2-thione;3-methylbenzo[d]oxazole-2(3H)-thione;N-Methylbenzoxazolinthion;3-Methyl-benzoxazolthion;3-Methyl-1,3-benzoxazole-2(3H)-thione;3-methyl-1,3-benzoxazole-2-thione
CAS
13673-63-7
化学式
C8H7NOS
mdl
MFCD00459943
分子量
165.216
InChiKey
VMOIPIVQHVFUFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:132-135
-
沸点:272.25°C (rough estimate)
-
密度:1.1979 (rough estimate)
-
溶解度:14.9 [ug/mL]
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:44.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S22,S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2934999090
-
危险性防范说明:P261,P264,P271,P280,P302+P352,P304+P340+P312,P305+P351+P338,P332+P313,P337+P313,P362,P403+P233,P405,P501
-
危险性描述:H315,H319,H335
-
储存条件:存于阴凉干燥处。
SDS
| Name: | 3-Methyl-2(3H)-benzoxazolethione 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 13673-63-7 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 13673-63-7 | 3-Methyl-2(3H)-benzoxazolethione | 97% | unlisted |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 13673-63-7: Personal Protective Equipment Eyes: Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Needles
Color: light brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 132-135 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H7NOS
Molecular Weight: 165.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, oxides of sulfur, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 13673-63-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-Methyl-2(3H)-benzoxazolethione - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 13673-63-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 13673-63-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 13673-63-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-巯基苯并恶唑 2-sulfanyl-1,3-benzoxazole 2382-96-9 C7H5NOS 151.189 3-甲基-2-苯并恶唑酮 3-methylbenzo[d]oxazol-2(3H)-one 21892-80-8 C8H7NO2 149.149 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-methyl-2-thioxo-2,3-dihydrobenzo[d]oxazole-6-carbaldehyde —— C9H7NO2S 193.226
反应信息
-
作为反应物:描述:3-甲基-1,3-苯并噁唑-2(3H)-硫酮 在 sodium triacetoxyborohydride 、 溶剂黄146 、 三氟乙酸 作用下, 以 甲醇 、 1,2-二氯乙烷 为溶剂, 生成 6-((((3R,4R,6S)-6-benzhydryl-4-hydroxytetrahydro-2H-pyran-3-yl)amino)methyl)-3-methylbenzo[d]oxazole-2(3H)-thione参考文献:名称:Substituted Pyran Derivatives摘要:提供了在单胺转运系统上表现出强效活性的某些3,6-二取代和2,4,5-三取代吡喃衍生物。这些3,6和2,4,5吡喃物在探究它们与单胺转运体系的结合效应以及与影响中枢神经系统的各种疾病之间的关系,或作为治疗涉及单胺转运和相关系统的各种中枢神经系统相关疾病的方法中是有用的。公开号:US20140309427A1
-
作为产物:描述:参考文献:名称:Desai et al., Journal of the Chemical Society, 1934, p. 1186,1190摘要:DOI:
文献信息
-
FUNGICIDAL MIXTURES申请人:Gregory Vann公开号:US20100240619A1公开(公告)日:2010-09-23Disclosed is a fungicidal composition comprising (a) at least one compound selected from the compounds of Formula 1 N-oxides, and salts thereof, wherein R 1 , R 2 , A, G, W, Z 1 , X, J, and n are as defined in the disclosure, and (b) at least one additional fungicidal compound. Also disclosed is a method for controlling plant diseases caused by fungal plant pathogens comprising applying to the plant or portion thereof, or to the plant seed, a fungicidally effective amount of the aforesaid composition. Also disclosed is a composition comprising component (a) of aforesaid composition and at least one insecticide. Also disclosed are compounds of Formula 1A, 1B and 1C, wherein R 1 , R 2 , A, G, W, Z 1 , X, J, n, Z 3 , M and J 1 are as defined in the disclosure.公开了一种含真菌化合物,包括:(a)至少一种选自公式1 N-氧化物的化合物,以及它们的盐类,其中R1,R2,A,G,W,Z1,X,J和n如公开所述定义,以及(b)至少一种额外的含真菌化合物。还公开了一种控制由真菌植物病原体引起的植物病害的方法,包括将有效量的前述组合物施用于植物或其部分,或施用于植物种子。还公开了一种组合物,包括前述组合物的组分(a)和至少一种杀虫剂。还公开了公式1A,1B和1C的化合物,其中R1,R2,A,G,W,Z1,X,J,n,Z3,M和J1如公开所述定义。
-
Photoaddition of benzoxazole-2-thiones with alkenes作者:Takehiko Nishio、Yo-ichi Mori、Ikuo Iida、Akira HosomiDOI:10.1039/p19960000921日期:——The photochemical reactions of the benzoxazole-2-thiones 6 have been examined. Irradiation of 3-unsubstituted benzoxazole-2-thione 6a in the presence of alkenes 2 gave 2-alkylated benzoxazoles 7, 9–15 and 8 (in the case of 2a). Irradiation of 3-substituted benzoxazole-2-thiones 6b–e and acrylonitrile 2f yielded the 2-alkylidenebenzoxazoles 17, 22 and 24–25. Irradiation of 6b–e in the presence of 1,1-di-and tetra-substituted alkenes 2a,c and e gave the amide derivatives 18–20, 23 and 26. The 3-(alk-ω-enyl)-benzoxazole-2-thiones 6g–i gave the lactam derivatives 28g–i upon irradiation. The formation of these photoproducts can be explained in terms of the intermediacy of amino spiro-thietanes, which are derived by [2 + 2] photocycloaddition of the CS bond of benzoxazole-2-thiones and the CC bond of alkenes.
-
[EN] SUBSTITUTED AROMATIC N-HETEROCYCLIC COMPOUNDS AS INHIBITORS OF MITOGEN-ACTIVATED PROTEIN KINASE INTERACTING KINASE 1 (MNK1) AND 2 (MNK2)<br/>[FR] COMPOSÉS N-HÉTÉROCYCLIQUES AROMATIQUES SUBSTITUÉS EN TANT QU'INHIBITEURS DE PROTÉINE KINASE ACTIVÉE PAR MITOGÈNE INTERAGISSANT AVEC LA KINASE 1 (MNK1) ET LA KINASE 2 (MNK2)申请人:UNIV NORTHWESTERN公开号:WO2017075367A1公开(公告)日:2017-05-04Disclosed are substituted aromatic N-heterocyclic compounds. The disclosed compounds typically exhibit kinase inhibition activity, for example, and inhibit Mnk1 kinase and/or Mnk2 kinase. The disclosed compounds may be used in pharmaceutical compositions and methods for treating diseases or disorders associated with Mnk1 kinase activity and/or Mnk2 kinase activity, such as cancers, diabetes, autism, and fragile X syndrome.揭示了替代芳香族N-杂环化合物。所述化合物通常表现出激酶抑制活性,例如,抑制Mnk1激酶和/或Mnk2激酶。所述化合物可用于制备药物组合物和治疗与Mnk1激酶活性和/或Mnk2激酶活性相关的疾病或紊乱的方法,如癌症、糖尿病、自闭症和脆性X综合征。
-
Quinolines and nitrogenated derivative therof substituted in 4-position by a piperidine-containing moiety and their use as antibacterial agents
-
Quinolines and nitrogenated derivative thereof substituted in 4-position by a piperidine-containing moiety and their use as antibacterial agents
表征谱图
-
氢谱1HNMR
-
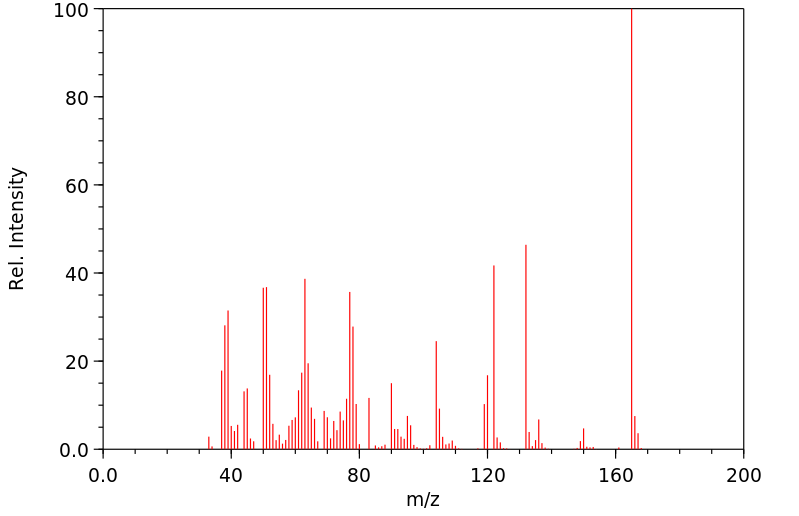
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(N-{4-[(6-溴-2-氧代-1,3-苯并恶唑-3(2H)-基)磺酰基]苯基}乙酰胺)
钙离子载体A23187半镁盐
钙离子载体A23187半钙盐
萘并[2,3-d]噁唑-2,8(3H,5H)-二酮,6,7-二氢-5-甲基-
萘并[2,3-d]噁唑-2,5-二酮,3,6,7,8-四氢-3,8-二甲基-
荧光增白剂EBF
苯并恶唑胺
苯并恶唑的取代物
苯并恶唑甲磺酰氯
苯并恶唑基-2-甲酰基-S-乙基-异缩氨基硫脲
苯并恶唑-2-羧酸酰肼
苯并恶唑-2-磺酸
苯并恶唑-2-甲酸
苯并恶唑-2-甲磺酸钠
苯并恶唑-2-乙酸
苯并恶唑
苯并噁唑-5-甲酸
苯并噁唑-2-羧酸乙酯
苯并噁唑-2-甲醛
苯并噁唑,5,7-二(1,1-二甲基乙基)-2-乙烯基-
苯并噁唑,5,7-二(1,1-二甲基乙基)-2-乙基-
苯并噁唑,4,7-二氯-2-(氯甲基)-
苯并噁唑,2-叠氮-
苯并噁唑,2-(氯甲基)-4,7-二氟-
苯并[d]恶唑-7-甲酸甲酯
苯并[d]恶唑-5-硼酸频哪醇酯
苯并[d]噁唑-6-甲醛
苯并[d]噁唑-2-羧酸甲酯
苯并[d]噁唑-2-甲醇
苯并[D]恶唑-7-胺
苯并[D]噁唑-4-基氨基甲酸叔丁酯
苯并[D]噁唑-2-羧酸钾
苯并-13C6-噁唑
离子载体
碘化二氢2-[3-(5,6-二氯-1,3-二乙基-1,3--2H-苯并咪唑-2-亚基)丙-1-烯基]-3-乙基-5-苯基苯并噁唑正离子
硫代偏糖醛
甲酰胺,N-乙基-N-[6-[(3-甲酰基苯氧基)甲基]-2-苯并噁唑基]-
甲酰胺,N-[6-(溴甲基)-2-苯并噁唑基]-N-乙基-
甲基硫酸1-甲基-8-[(甲基氨基甲酰)氧代]喹啉正离子
甲基6-氨基-1,3-苯并恶唑-2-羧酸酯
甲基2-氨基-1,3-苯并恶唑-5-羧酸酯
甲基1,3-苯并恶唑-2-基乙酸酯
甲基-2-乙基-1,3-苯并唑-5-羧酸乙酯
甲基-1,3-苯并唑-5-羧酸乙酯
环戊二烯并[e][1,3]恶嗪-5,6-二胺
环戊二烯并[d][1,3]恶嗪-6,7-二胺
溴氯唑酮
溴化二氢2-[3-[1-[4-[(乙酰氨基)磺基基]丁基]-5,6-二氯-3-乙基-1,3--2H-苯并咪唑-2-亚基]丙-1-烯基]-3-乙基-5-苯基苯并噁唑正离子
氰基二硫代亚氨酸(6-氯-2-氧代-3(2H)-苯并恶唑基)甲基甲基酯
氰基-二硫代亚氨酸甲基(2-氧代-3(2H)-苯并恶唑基)甲基酯