L-瓜氨酸 | 372-75-8
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:214 °C
-
比旋光度:25.5 º (c=8, 6N HCl)
-
沸点:306.48°C (rough estimate)
-
密度:1.2919 (rough estimate)
-
溶解度:酸水溶液(微溶)。水(微溶)
-
LogP:-3.190
-
物理描述:Solid
-
碰撞截面:138 Ų [M+H]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-4.3
-
重原子数:12
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:118
-
氢给体数:4
-
氢受体数:4
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S24/25
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29241900
-
危险品运输编号:OTH
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:储存于阴凉干燥处,密封保存。
SDS
| Name: | L-Citrulline Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 372-75-8 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 372-75-8 | L-Citrulline | 100 % | 206-759-6 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid if cough or other symptoms appear.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 372-75-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 220 deg C (decomposes)
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Soluble in water.
Specific Gravity/Density: Not available.
Molecular Formula: C6H13N3O3
Molecular Weight: 175.1093
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Strong oxidants.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 372-75-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
L-Citrulline - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 372-75-8: 0
Canada
CAS# 372-75-8 is listed on Canada's NDSL List.
CAS# 372-75-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 372-75-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
白色结晶粉末,易溶于水,难溶于甲醇和乙醇。能被磷钨酸从其浓溶液中沉淀出来,与浓碱溶液作用生成鸟氨酸、二氧化碳和氨。其铜盐为蓝色棱柱晶体,熔点257-258℃。其氢氧化物的熔点185℃ (分解),在水中[α]D22为+17.9°。存在于西瓜等瓜果中。人体中的瓜氨酸是一种非蛋白质氨基酸,不参与蛋白质合成,但具有肽键形成能力,过去认为仅存在于鸟氨酸-尿素循环的中间产物。
应用L-瓜氨酸拥有多种重要的生理功能,如清除自由基、异体排斥效应指示剂、血管舒张作用、稳定血压及诊断类风湿关节炎和抗氧化等。它还有显著恢复精力和提高人体免疫力的作用。近年来,L-瓜氨酸在食品、化妆品和药物领域越来越受到重视,并具有广泛的应用开发前景。
功效与作用瓜氨酸是一种非蛋白质氨基酸,具有肽键形成能力但不参与蛋白质合成。研究发现,瓜氨酸曾一度被认为仅是尿素循环的中间产物,但实际上它广泛应用于医药、食品和保健等领域,拥有良好的药理作用。
-
防治前列腺疾病 瓜氨酸对防治前列腺炎、前列腺肿胀、前列腺肥大及增生症和前列腺癌具有明显效果。王江海等人发明了通过诱导南瓜子胚细胞萌动来提升瓜氨酸含量及其抗增生因子活性物质的提取方法,可以作为医药原料或功能性保健食品。
-
提高男性性功能 瓜氨酸能促使人体产生氮氧化物,这种物质对男性的性能力至关重要。它不仅可以完全被血液吸收,更容易生成氮氧化物使阴茎勃起,而且作为一种纯天然产品无副作用,有助于增强男子身体状况,被誉为“绿色伟哥”。近年来已有多家公司开发出相关产品,例如从西瓜中提取的瓜氨酸制成片剂和胶囊。
-
抗衰老与增强免疫力 研究表明,瓜氨酸具有较强的抗氧化能力,能清除羟基、增加NO合成所需的精氨酸,有效保护DNA及PMN免受氧化反应侵害。因此,瓜氨酸作为抗衰老和提高免疫力的保健品或女性美容化妆品,具备护肤防皱、祛斑抗衰老的功效。例如L-瓜氨酸苹果酸盐已成为国际市场上销售量增长较快的一种氨基酸盐类保健产品。
白色结晶粉末,由于含有极性侧链而易溶于水,难溶于甲醇和乙醇。
用途L-瓜氨酸的主要功效包括:提高免疫系统功能;维护关节运动机能;平衡正常血糖水平;抗氧化,吸收有害自由基;维持胆固醇正常水平;维护健康肺功能;提高脑力清晰度;降低压力与克服沮丧情绪;增强健康性生活质量。利用NO合成酶,将左型精氨酸转化为氮氧化物的中间体。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 DL-瓜氨酸 citrulline 627-77-0 C6H13N3O3 175.188 L-鸟氨酸 L-ornithine 70-26-8 C5H12N2O2 132.162 (2S)-2-氨基-5-[(N'-丙-2-烯基甲脒基)氨基]戊酸 Nω-allyl-L-arginine 139461-37-3 C9H18N4O2 214.268 L-精氨酸 L-arginine 74-79-3 C6H14N4O2 174.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-amino-5-ureidopentanoic acid methyl ester —— C7H15N3O3 189.214 —— L-(+)-citrulline ethyl ester 80822-58-8 C8H17N3O3 203.241 —— 4-hydroxy-L-citrulline 3618-90-4 C6H13N3O4 191.187 —— Nα-carbamoyl-(S)-citrulline 117920-63-5 C7H14N4O4 218.213 L-鸟氨酸 L-ornithine 70-26-8 C5H12N2O2 132.162 —— 5-Aminocarbonylamino-2-oxopentansaeure 52717-00-7 C6H10N2O4 174.156 —— (2S)-5-(carbamoylamino)-2-hydroxypentanoic acid 413622-19-2 C6H12N2O4 176.172 L-精氨酸 L-arginine 74-79-3 C6H14N4O2 174.203
反应信息
-
作为反应物:描述:参考文献:名称:Photocatalytic one-step syntheses of cyclic imino acids by aqueous semiconductor suspensions摘要:DOI:10.1021/jo00308a005
-
作为产物:参考文献:名称:线粒体和线粒体中的纳特维氏菌摘要:DOI:10.1002/hlca.19490320707
文献信息
-
Cobalamin conjugates for anti-tumor therapy申请人:Weinshenker M. Ned公开号:US20050054607A1公开(公告)日:2005-03-10The present invention provides a cobalamin-drug conjugate suitable for the treatment of tumor related diseases. Cobalamin is indirectly covalently bound to an anti-tumor drug via a cleavable linker and one or more optional spacers. Cobalamin is covalently bound to a first spacer or the cleavable linker via the 5′-OH of the cobalamin ribose ring. The drug is bound to a second spacer of the cleavable linker via an existing or added functional group on the drug. After administration, the conjugate forms a complex with transcobalamin (any of its isoforms). The complex then binds to a receptor on a cell membrane and is taken up into the cell. Once in the cell, an intracellular enzyme cleaves the conjugate thereby releasing the drug. Depending upon the structure of the conjugate, a particular class or type of intracellular enzyme affects the cleavage. Due to the high demand for cobalamin in growing cells, tumor cells typically take up a higher percentage of the conjugate than do normal non-growing cells. The conjugate of the invention advantageously provides a reduced systemic toxicity and enhanced efficacy as compared to a corresponding free drug.本发明提供了一种适用于治疗肿瘤相关疾病的钴胺素-药物结合物。钴胺素通过可切割的连接剂间接共价结合到抗肿瘤药物上,还可以通过一个或多个可选的间隔物。钴胺素通过其核糖环的5'-OH与第一间隔物或可切割连接剂共价结合。药物通过其现有或添加的功能基团与可切割连接剂的第二间隔物结合。在给药后,结合物与转钴胺素(其任何同工异构体)形成复合物。然后,该复合物结合到细胞膜上的受体并被细胞摄取。一旦进入细胞,细胞内酶将切割结合物,从而释放药物。根据结合物的结构,特定类别或类型的细胞内酶影响切割。由于生长细胞对钴胺素的需求量较高,肿瘤细胞通常摄取结合物的比例高于正常非生长细胞。本发明的结合物与相应的游离药物相比,具有较低的全身毒性和增强的疗效。
-
[EN] POLYCONJUGATES FOR DELIVERY OF RNAI TRIGGERS TO TUMOR CELLS IN VIVO<br/>[FR] POLYCONJUGUÉS POUR L'ADMINISTRATION DE DÉCLENCHEURS D'ARNI À DES CELLULES TUMORALES IN VIVO申请人:ARROWHEAD RES CORP公开号:WO2015021092A1公开(公告)日:2015-02-12The present invention is directed compositions for delivery of RNA interference (RNAi) triggers to integrin positive tumor cells in vivo. The compositions comprise RGD ligand- targeted amphipathic membrane active polyamines reversibly modified with enzyme cleavable dipeptide-amidobenzyl-carbonate masking agents. Modification masks membrane activity of the polymer while reversibility provides physiological responsiveness. The reversibly modified polyamines (dynamic polyconjugate or conjugate) are further covalently linked to an RNAi trigger.
-
Small molecules for treatment of hypercholesterolemia and related diseases申请人:Sircar C. Jagadish公开号:US20050277690A1公开(公告)日:2005-12-15The present invention provides compositions adapted to enhance reverse cholesterol transport in mammals. The compositions are suitable for oral delivery and useful in the treatment and/or prevention of hypercholesterolemia, atherosclerosis and associated cardiovascular diseases.
-
Alpha-Ketoamide Inhibitors Of Cysteine Proteases申请人:Phelix Therapeutics公开号:US20190085024A1公开(公告)日:2019-03-21The disclosure provides compounds comprising a α-ketoamide linkage that is terminated on each end by an amino acid, such as compounds of Formula (I), wherein RA-RC are defined herein. Also provided are compositions containing these compounds and methods of inhibiting calpain activity, treating a calpain-mediated disorder, inhibiting cathepsin-B, cathepsin-L, cathepsin-S, or cathepsin-L activity, and methods of treating a cathepsin-B, cathepsin-L, cathepsin-S, or cathepsin-L mediated disorder using these compounds and compositions.
-
THIOL-ENE CLICK CHEMISTRY FOR DRUG CONJUGATES申请人:SYSCHEM, INC.公开号:US20130323169A1公开(公告)日:2013-12-05The present invention relates to linker molecules that readily conjugate cellular recognition ligand at one end and drug payload at the other, and are useful in treating or preventing cancer, an autoimmune disease, an inflammatory condition, a central nervous system disorder or an infection. The linker inker molecules of the invention are represented by Formula I, II and III; Linker-Drug compounds represented by Formula IV, V and VI; and Ligand-Linker-Drug conjugates represented by Formula VII, VIII and IX:
表征谱图
-
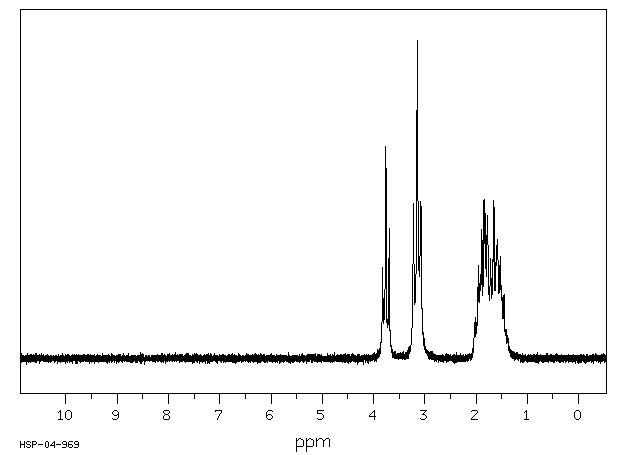
氢谱1HNMR
-
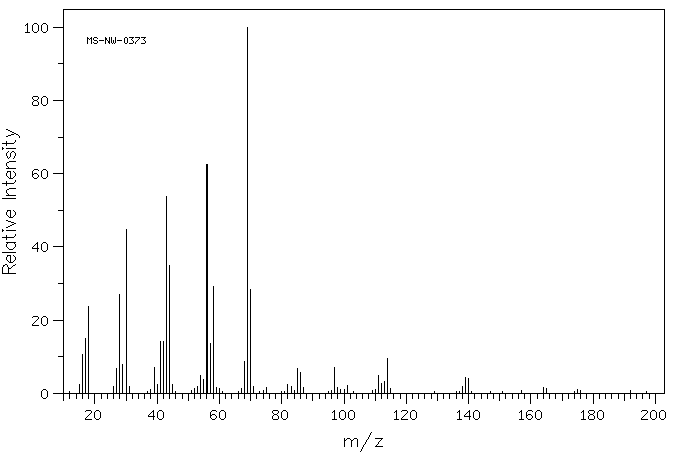
质谱MS
-
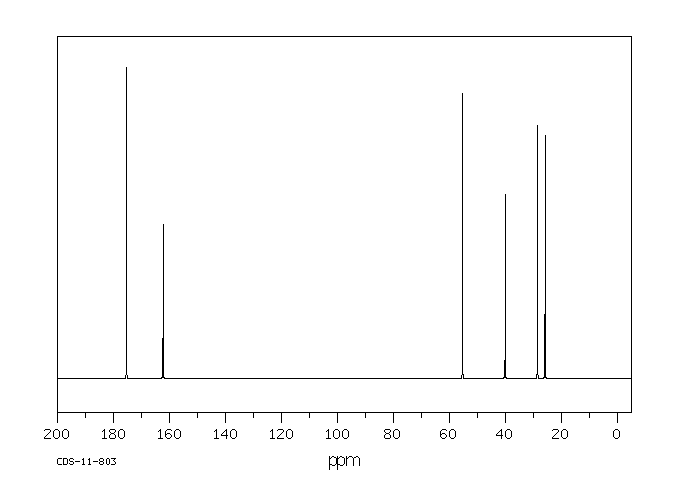
碳谱13CNMR
-
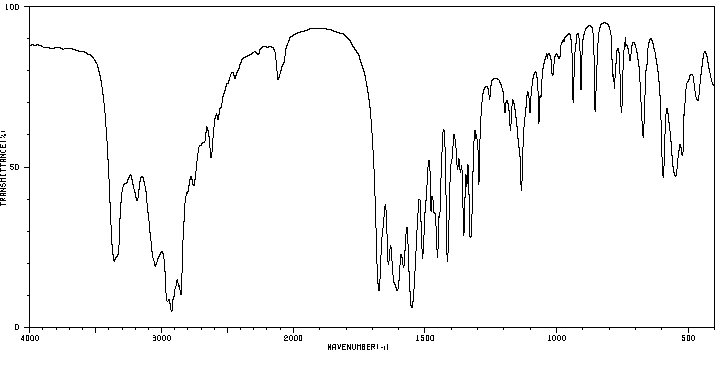
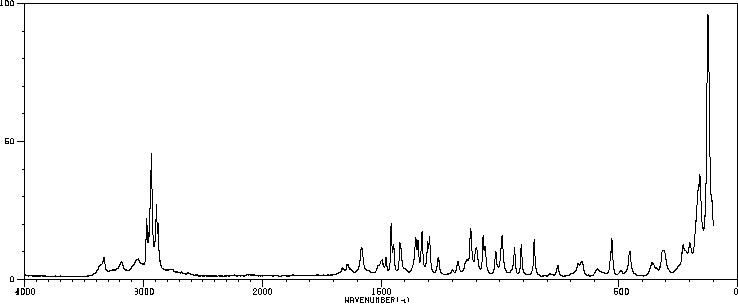
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息