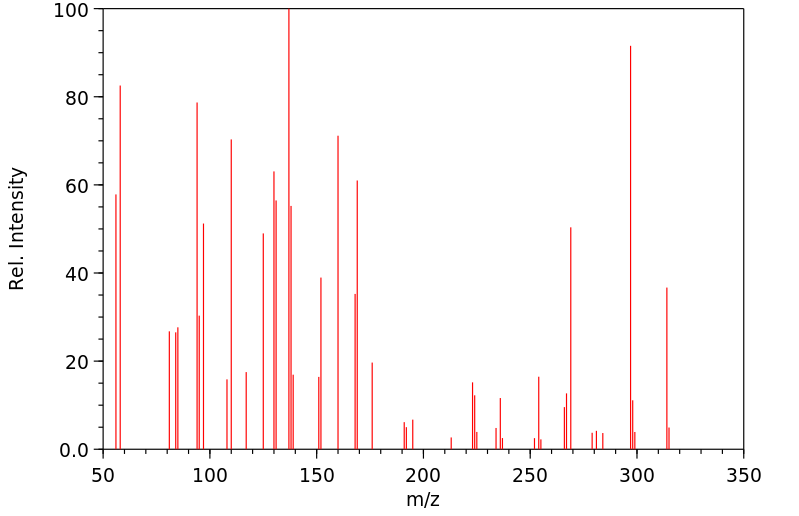
代谢
Ranitidine undergoes significant first-pass metabolism after oral admin. It is metabolized in the liver to the pharmacologically inactive desmethylranitidine, ranitidine-N-oxide, and ranitidine-S-oxide.
来源:Hazardous Substances Data Bank (HSDB)