4-氯苯磺酸 | 98-66-8
中文名称
4-氯苯磺酸
中文别名
對氯苯磺酸;对氯苯磺酸
英文名称
4-chloro-benzenesulfonic acid
英文别名
4-chlorobenzenesulfonate;p-chlorobenzenesulfonic acid;4-Chlorobenzenesulfonic acid
CAS
98-66-8
化学式
C6H5ClO3S
mdl
MFCD00065342
分子量
192.623
InChiKey
RJWBTWIBUIGANW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:102 °C
-
沸点:149 °C/22 mmHg (lit.)
-
密度:1.449 (estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶)
-
稳定性/保质期:
稳定存放,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.6
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:62.8
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:8
-
危险品标志:C
-
安全说明:S24/25,S26,S27,S28,S36/37/39,S45
-
危险类别码:R22,R34
-
WGK Germany:3
-
海关编码:2904909090
-
危险品运输编号:UN 2583 8/PG 2
-
危险类别:8
-
RTECS号:DB5074000
-
包装等级:III
-
危险性防范说明:P261,P280,P305+P351+P338,P310
-
危险性描述:H302,H314
-
储存条件:存放于惰性气体中,避免接触湿气(吸湿)。
SDS
| Name: | 4-Chlorobenzenesulfonic acid (techn) 90% (titr.) Material Safety Data Sheet |
| Synonym: | |
| CAS: | 98-66-8 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 98-66-8 | 4-Chlorobenzenesulfonic acid (techn) | 90.0 | 202-690-0 |
Risk Phrases: 22 34
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed. Causes burns.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. Aspiration may lead to pulmonary edema. May cause systemic effects.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed.
Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Do not ingest or inhale. Use with adequate ventilation. Discard contaminated shoes.
Storage:
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 98-66-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Flakes
Color: gray-beige
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 102 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H5ClO3S
Molecular Weight: 192.62
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 98-66-8: DB5074000 LD50/LC50:
CAS# 98-66-8: Oral, rat: LD50 = >500 mg/kg.
Carcinogenicity:
4-Chlorobenzenesulfonic acid (techn) - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ALKYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing Group: III
IMO
Shipping Name: ALKYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing Group: III
RID/ADR
Shipping Name: ALKYLSULPHONIC ACIDS, SOLID
Hazard Class: 8
UN Number: 2585
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: C
Risk Phrases:
R 22 Harmful if swallowed.
R 34 Causes burns.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 98-66-8: No information available.
Canada
CAS# 98-66-8 is listed on Canada's NDSL List.
CAS# 98-66-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 98-66-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
针状结晶。熔点为67℃(也有报道为68℃),沸点在147-148℃(3.33kPa)之间。它能溶于水和乙醇。
用途
主要用于医药和染料中间体领域。
生产方法
(1)由对氨基苯磺酸制备:将对氨基苯磺酸与盐酸加入水中,冷却至20℃以下后滴加亚硝酸钠溶液,反应至终点得到重氮液。另外,将硫酸铜与氯化钠溶于约80℃的水温中,待其完全溶解后再滴加碱性亚硫酸钠溶液,并搅拌反应20分钟后冷却至室温,静置沉淀。将沉淀用倾泻法清洗并溶解在盐酸中,冷却至0℃以下后滴加上述重氮液生成对氯苯磺酸。接着,将反应液升温至80℃,加入食盐进行盐析处理,过滤即得对氯苯磺酸钠。
(2)由氯苯磺化制备:在反应锅内加入98%的浓硫酸和10%发烟硫酸,并搅拌15分钟,随后逐渐加入干燥的氯苯,在95-100℃条件下搅拌反应5小时。检查磺化终点以确保反应物酸度不大于70%,且能在水中完全溶解。
类别
有毒物质
毒性分级
中毒级别
急性毒性
口服-大鼠 LD50: > 500 毫克/公斤
储运特性
库房应低温、通风且干燥保存
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对氯苯亚磺酸 4-chlorobenzenesulfinic acid 100-03-8 C6H5ClO2S 176.624 4-氯苯硫甲酯 methyl 4-chlorobenzenesulfonate 15481-45-5 C7H7ClO3S 206.65 4-氯苯磺酸乙酯 ethyl p-chlorobenzenesulfonate 20443-71-4 C8H9ClO3S 220.677 3-氯-苯磺酸 3-chlorobenzenesulphonic acid 20677-52-5 C6H5ClO3S 192.623 —— 4-Chlor-benzolsulfonsaeure-allylester 6165-74-8 C9H9ClO3S 232.688 2-氯-苯磺酸 2-chlorobenzenesulfonic acid 27886-58-4 C6H5ClO3S 192.623 —— phenyl 4-chlorobenzenesulfonate 2437-33-4 C12H9ClO3S 268.721 杀螨酯 chlorofensone 80-33-1 C12H8Cl2O3S 303.166 4-氯苯磺酰胺 4-Chlorobenzenesulfonamide 98-64-6 C6H6ClNO2S 191.638 —— 4-chloro-benzenesulfonic acid p-tolyl ester 37100-89-3 C13H11ClO3S 282.748 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-氯苯硫甲酯 methyl 4-chlorobenzenesulfonate 15481-45-5 C7H7ClO3S 206.65 苯磺酸 benzenesulfonic acid 98-11-3 C6H6O3S 158.178 4-氯苯磺酸乙酯 ethyl p-chlorobenzenesulfonate 20443-71-4 C8H9ClO3S 220.677 3-氯-苯磺酸 3-chlorobenzenesulphonic acid 20677-52-5 C6H5ClO3S 192.623 —— isopropyl p-chlorobenzenesulfonate 69564-62-1 C9H11ClO3S 234.704 —— 4-chloro-benzenesulfonic acid-(2-bromo-ethyl ester) 61855-71-8 C8H8BrClO3S 299.573 2-氯-苯磺酸 2-chlorobenzenesulfonic acid 27886-58-4 C6H5ClO3S 192.623 —— p-chlorobenzenesulfonic anhydride 14248-65-8 C12H8Cl2O5S2 367.231 4-羟基苯磺酸 p-hydoroxybenzenesulfonic acid 98-67-9 C6H6O4S 174.177 对氨基苯磺酸 4-aminobenzene sulfonic acid 121-57-3 C6H7NO3S 173.192 —— phenyl 4-chlorobenzenesulfonate 2437-33-4 C12H9ClO3S 268.721 4-氯苯磺酸苄酯 Phenylmethan-sulfonsaeure-p-chlorphenylester 13086-79-8 C13H11ClO3S 282.748 —— 5-chloro-benzene-1,3-disulfonic acid 617-98-1 C6H5ClO6S2 272.687 —— 2-Oxopentyl 4-chlorobenzenesulfonate 80520-45-2 C11H13ClO4S 276.741 4-氯-苯磺酰氟 p-chlorobenzenesulfonyl fluoride 349-89-3 C6H4ClFO2S 194.614 —— 2-oxoundecyl-p-chlorobenzene sulfonate 80519-87-5 C17H25ClO4S 360.902 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:DE205150摘要:公开号:
-
作为产物:描述:参考文献:名称:二氧化硅硫酸磺化芳环的新方法摘要:在1,2-二茂铁乙烷中或在无溶剂条件下,用二氧化硅硫酸对芳香族化合物进行直接和化学选择性磺化。DOI:10.1016/j.tetlet.2004.07.023
-
作为试剂:描述:二(2-氯乙基)胺盐酸盐 、 2,3-二氯苯胺 在 4-氯苯磺酸 作用下, 以 5,5-dimethyl-1,3-cyclohexadiene 为溶剂, 以87.9 %的产率得到1-(2,3-二氯苯基)哌嗪参考文献:名称:一种制备1-(2,3-二氯苯基)哌嗪盐酸盐的方法摘要:本发明公开了一种化合物1‑(2,3‑二氯苯基)哌嗪盐酸盐的合成方法,该方法以2,3‑二氯苯胺和双(2‑氯乙基)胺盐酸盐为原料,经过催化剂催化缩合反应得到1‑(2,3‑二氯苯基)哌嗪盐酸盐。本发明合成方法简单,可以高转化率得到产物,且反应安全性高;本发明所用原料环保压力较低,反应条件温和,对设备的要求较低,适合工业化生产要求。公开号:CN116199644A
文献信息
-
[EN] NOVEL COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR THE TREATMENT OF INFLAMMATORY DISORDERS<br/>[FR] NOUVEAUX COMPOSÉS ET COMPOSITIONS PHARMACEUTIQUES LES COMPRENANT POUR LE TRAITEMENT DE TROUBLES INFLAMMATOIRES申请人:GALAPAGOS NV公开号:WO2017012647A1公开(公告)日:2017-01-26The present invention discloses compounds according to Formula (I), wherein R1, R3, R4, R5, L1, and Cy are as defined herein. The present invention also provides compounds, methods for the production of said compounds of the invention, pharmaceutical compositions comprising the same and their use in allergic or inflammatory conditions, autoimmune diseases, proliferative diseases, transplantation rejection, diseases involving impairment of cartilage turnover, congenital cartilage malformations, and/or diseases associated with hypersecretion of IL6 and/or interferons. The present invention also methods for the prevention and/or treatment of the aforementioned diseases by administering a compound of the invention.本发明公开了根据式(I)的化合物,其中R1、R3、R4、R5、L1和Cy如本文所定义。本发明还提供了该发明的化合物、制备该化合物的方法、包括相同化合物的药物组合物以及它们在过敏或炎症症状、自身免疫疾病、增殖性疾病、移植排斥、涉及软骨周转障碍的疾病、先天软骨畸形和/或与IL6和/或干扰素过度分泌相关的疾病中的使用。本发明还提供了通过给予该发明的化合物来预防和/或治疗上述疾病的方法。
-
Novel compounds and compositions as protease inhibitors申请人:——公开号:US20020052378A1公开(公告)日:2002-05-02The present invention relates to novel cysteine protease inhibitors of Formula I: 1 the pharmaceutically acceptable salts and N-oxide derivatives thereof, their use as therapeutic agents and methods of making them.
-
Ataxia Telengiectasia And RAD3-Related (ATR) Inhibitors And Methods Of Their Use申请人:Atrin Pharmaceuticals LLC公开号:US20170291911A1公开(公告)日:2017-10-12The disclosure is directed to compounds and compositions that inhibit Ataxia Telengiectasia And Rad3-Related (ATR) Protein Kinase and methods of their use.该披露涉及抑制共济失调毛细血管扩张性和Rad3相关蛋白激酶(ATR)的化合物和组合物,以及它们的使用方法。
-
[EN] TGR5 AGONISTS<br/>[FR] AGONISTES DE TGR5申请人:EXELIXIS INC公开号:WO2011071565A1公开(公告)日:2011-06-16TGR5 agonists of structural formula VIII(Q), wherein X, R1, R2, and R5 are defined in the specification, pharmaceutically acceptable salts thereof, compositions thereof, and use of the compounds and compositions for treating diseases. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases.TGR5激动剂的结构式VIII(Q),其中X、R1、R2和R5在规范中定义,其药用盐、组合物以及用于治疗疾病的化合物和组合物的使用。该发明还包括在制药品中使用这些化合物以及用于制造药物,特别是用于治疗疾病。
-
[EN] TRIAZOLE AND IMIDAZOLE DERIVATIVES FOR USE AS TGR5 AGONISTS IN THE TREATMENT OF DIABETES AND OBESITY<br/>[FR] DÉRIVÉS DE TRIAZOLE ET D'IMIDAZOLE DESTINÉS À ÊTRE UTILISÉS EN TANT QU'AGONISTES DE TGR5 DANS LE TRAITEMENT DU DIABÈTE ET DE L'OBÉSITÉ申请人:EXELIXIS INC公开号:WO2010093845A1公开(公告)日:2010-08-19The present invention comprises TGR5 agonists of structural formula I, wherein X, R1, R2, and R5 are defined herein, as well as N-oxides of them and pharmaceutically acceptable salts thereof. The invention further comprises composition comprising the compounds, N-oxides, and/or pharmaceutically acceptable salts thereof. The invention also comprises use of the compounds and compositions for treating diseases in which TGR5 is a mediator or is implicated. The invention also comprises use of the compounds in and for the manufacture of medicaments, particularly for treating diseases in which TGR5 is a mediator or is implicated.本发明包括结构式I的TGR5激动剂,其中X、R1、R2和R5在此处定义,以及它们的N-氧化物和其药学上可接受的盐。该发明还包括包含这些化合物、N-氧化物和/或其药学上可接受的盐的组合物。该发明还包括利用这些化合物和组合物治疗TGR5是介质或涉及的疾病。该发明还包括利用这些化合物制造药物,特别是用于治疗TGR5是介质或涉及的疾病。
表征谱图
-
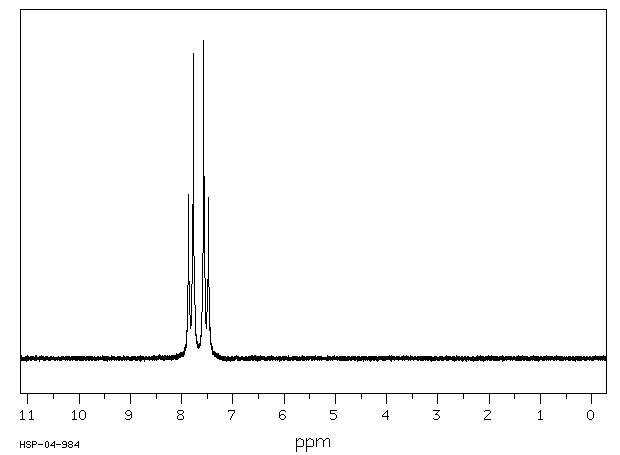
氢谱1HNMR
-
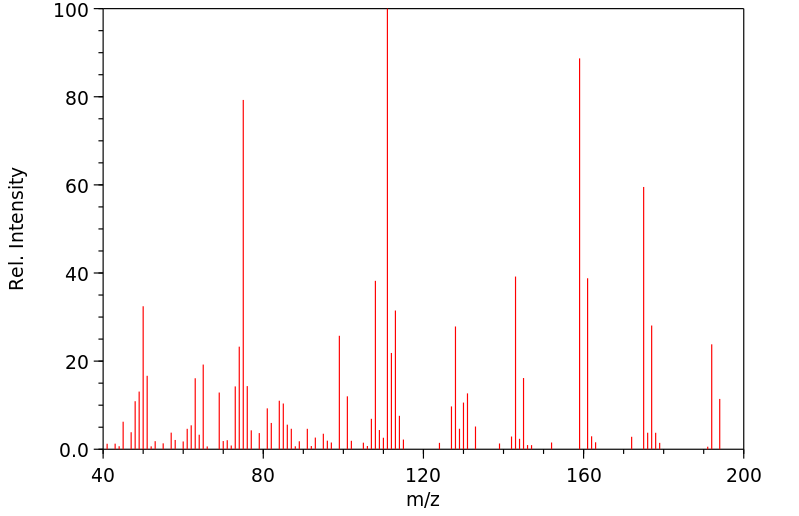
质谱MS
-
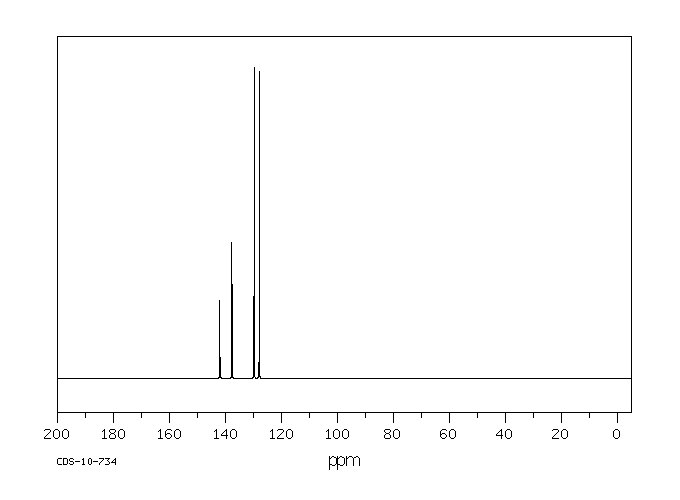
碳谱13CNMR
-
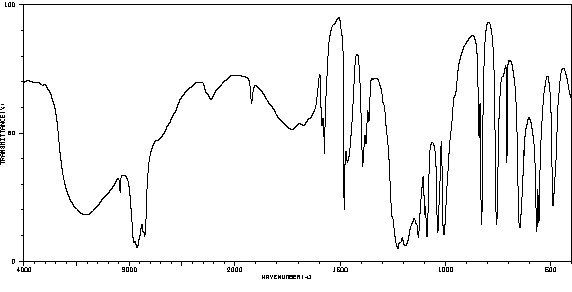
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫