氯甲酸正戊酯 | 638-41-5
中文名称
氯甲酸正戊酯
中文别名
氯甲酸戊酯
英文名称
pentyl chloroformate
英文别名
n-pentyl chloroformate;amyl chloroformate;n-amyl chloroformate;pentyl carbonochloridate;Chlorameisensaeure-pentylester;Chlorameisensaeure-n-pentylester;chloroformic acid n-pentyl ester
CAS
638-41-5
化学式
C6H11ClO2
mdl
——
分子量
150.605
InChiKey
XHRRYUDVWPPWIP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:161 °C
-
密度:1,04 g/cm3
-
闪点:53 °C
-
溶解度:可溶于氯仿、乙酸乙酯
-
LogP:3.03
-
物理描述:Liquid
-
保留指数:926
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.83
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:C
-
安全说明:S26,S36,S45
-
危险类别码:R10
-
海关编码:2915900090
-
危险品运输编号:3277
-
危险类别:6.1
-
包装等级:I
-
储存条件:储存注意事项: - 储存于阴凉、干燥、通风良好的库房。 - 远离火种、热源,库温不超过32℃,相对湿度不超过80%。 - 保持容器密封。 - 应与氧化剂、酸类、碱类、胺类等分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
Amyl Chloroformate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Amyl Chloroformate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Flammable liquids Category 3
HEALTH HAZARDS
Acute toxicity (Inhalation) Category 2
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Flammable liquid and vapour
Fatal if inhaled
Causes severe skin burns and eye damage
Precautionary statements
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Do not breathe.
Use only outdoors or in a well-ventilated area.
Wash hands thoroughly after handling.
Wear respiratory protection.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Immediately call a POISON CENTER or doctor/physician.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Amyl Chloroformate
Section 2. HAZARDS IDENTIFICATION
[Storage] Store in a well-ventilated place. Keep container tightly closed.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Amyl Chloroformate
>97.0%(GC)(T)
Percent:
CAS Number: 638-41-5
Chloroformic Acid Amyl Ester , Chloroformic Acid Pentyl Ester , Pentyl Chloroformate
Synonyms:
Chemical Formula: C6H11ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
Ingestion:
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Extinguishing media not to Water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water but NO direct contact with water. Eliminate all ignition sources if
safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
devices should be prepared in case of a fire. Use spark-proof tools and explosion-
hazards:
proof equipment.
Section 7. HANDLING AND STORAGE
Handling
Amyl Chloroformate
Section 7. HANDLING AND STORAGE
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapor or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
May develop pressure. Open carefully.
Storage
Storage conditions: Keep container tightly closed. Store in an explosion-poof refregerator.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: clear
Color: Colorless - Almost colorless
No data available
Odor:
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 161 °C
No data available
Flash Point:
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.03
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: Decomposes in contact with water and liberates toxic gases.
Conditions to avoid: Heat-sensitive, Moisture-sensitive
Incompartible materials: oxidizing agents, bases, water
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Amyl Chloroformate
Section 11. TOXICOLOGICAL INFORMATION
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 6.1: Toxic substance.
Subsidiary risk: 8: Corrosive.
UN-No: 3277
Proper shipping name: Chloroformates, toxic, corrosive, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Amyl Chloroformate
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Flammable liquids Category 3
HEALTH HAZARDS
Acute toxicity (Inhalation) Category 2
Category 1B
Skin corrosion/irritation
Serious eye damage/eye irritation Category 1
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Flammable liquid and vapour
Fatal if inhaled
Causes severe skin burns and eye damage
Precautionary statements
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Do not breathe.
Use only outdoors or in a well-ventilated area.
Wash hands thoroughly after handling.
Wear respiratory protection.
Wear protective gloves/eye protection/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Immediately call a POISON CENTER or doctor/physician.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Amyl Chloroformate
Section 2. HAZARDS IDENTIFICATION
[Storage] Store in a well-ventilated place. Keep container tightly closed.
Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Amyl Chloroformate
>97.0%(GC)(T)
Percent:
CAS Number: 638-41-5
Chloroformic Acid Amyl Ester , Chloroformic Acid Pentyl Ester , Pentyl Chloroformate
Synonyms:
Chemical Formula: C6H11ClO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
Ingestion:
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, carbon dioxide.
media:
Extinguishing media not to Water
be used:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water but NO direct contact with water. Eliminate all ignition sources if
safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (self-contained breathing apparatus). Keep
protective equipment and people away from and upwind of spill/leak. Ensure adequate ventilation. Entry to non-
emergency procedures: involved personnel should be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into an
containment and cleaning airtight container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Do not allow contact with water. Remove all sources of ignition. Fire-extinguishing
devices should be prepared in case of a fire. Use spark-proof tools and explosion-
hazards:
proof equipment.
Section 7. HANDLING AND STORAGE
Handling
Amyl Chloroformate
Section 7. HANDLING AND STORAGE
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapor or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapor or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
May develop pressure. Open carefully.
Storage
Storage conditions: Keep container tightly closed. Store in an explosion-poof refregerator.
Store under inert gas.
Protect from moisture.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Respiratory protection: Half or full facepiece respirator, self-contained breathing apparatus(SCBA), supplied
air respirator, etc. Use respirators approved under appropriate government standards
and follow local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Liquid
Physical state (20°C):
Form: clear
Color: Colorless - Almost colorless
No data available
Odor:
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: 161 °C
No data available
Flash Point:
Explosive limits
Lower: No data available
Upper: No data available
Density: 1.03
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: Decomposes in contact with water and liberates toxic gases.
Conditions to avoid: Heat-sensitive, Moisture-sensitive
Incompartible materials: oxidizing agents, bases, water
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Amyl Chloroformate
Section 11. TOXICOLOGICAL INFORMATION
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 6.1: Toxic substance.
Subsidiary risk: 8: Corrosive.
UN-No: 3277
Proper shipping name: Chloroformates, toxic, corrosive, n.o.s.
Packing group: II
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:铜(I)催化从叠氮基甲酸酯和芳基末端炔烃合成1,4-二取代的1,2,3-三唑摘要:铜(I)催化的叠氮化物-炔烃环加成反应已得到广泛研究,并广泛应用于有机合成中。然而,与电子不足的叠氮化物形成1,2,3-三唑是一个具有挑战性的问题。在本报告中,我们已经证明了使用市售的[Cu(CH 3 CN)在温和条件下的4 ] PF 6铜(I)催化剂。DOI:10.1021/acs.joc.8b00022
-
作为产物:参考文献:名称:N-羟基氨基甲酸酯的合成及一些反应摘要:羟胺和氯甲酸烷基酯在碱性介质中反应,以形成ñ - , - NO -二- ,和NNO -三烷氧羰基羟胺,依次。N-甲基羟胺类似地得到N-和NO-二烷氧基羰基-N-甲基-羟胺,O-甲基羟胺产生N-和NN-二烷氧基羰基-O-甲基羟胺。N-苯基羟胺与1当量的氯甲酸乙酯反应,得到N-羟基-N-苯基氨基甲酸酯。盐酸羟胺和氯甲酸乙酯产生产物,其变为羟基氨基甲酸酯。N-羟基氨基甲酸酯烷基和羟基脲得到O-黄羟基衍生物。所述Ñ -hydroxycarbamates用含水四氰二钾(II)而形成二钾tricyanonitrosylnickelate(II)。在这些羟氨基衍生物的酸和碱水解过程中,没有证据表明发生了洛森型重排。讨论了可能的水解机理。DOI:10.1039/j39660000346
-
作为试剂:描述:(2S,5R)-6-(苄氧基)-7-氧代-1,6-二氮杂双环[3.2.1]辛烷-2-羧酸 在 N-甲基吗啉 、 ammonium hydroxide 、 氯甲酸正戊酯 、 异辛酸钠 、 palladium 10% on activated carbon 、 氢气 、 三甲基铵三氧化硫共聚物 、 三乙胺 作用下, 以 乙醇 、 水 、 异丙醇 为溶剂, -25.0~40.0 ℃ 、500.01 kPa 条件下, 反应 0.5h, 生成 avibactam参考文献:名称:一种阿维巴坦钠的制备方法摘要:一种阿维巴坦钠的制备方法,它涉及医药领域,本发明的方法以(2S,5R)‑苄氧氨基哌啶‑2‑甲酸乙酯草酸盐起始原料与三光气有机溶剂和碱的存在下环合,并在氢化反应后采经三氧化硫络合物磺酸化后与铵离子源成盐后得到化合物制备阿维巴坦钠。该方法原料廉价易得,反应条件温和,操作简单,安全性更高,收率高,纯度好,适合规模化工业大生产。本发明应用于药物制备领域。公开号:CN114989169A
文献信息
-
Compounds and uses thereof for decreasing activity of hormone-sensitive lipase申请人:——公开号:US20030166644A1公开(公告)日:2003-09-04Use of compounds to inhibit hormone-sensitive lipase, pharmaceutical compositions comprising the compounds, methods of treatment employing these compounds and compositions, and novel compounds. The present compounds are inhibitors of hormone-sensitive lipase and may be useful in the treatment and/or prevention of medical disorders where a decreased activity of hormone-sensitive lipase is desirable.
-
[EN] INDAZOLE- AND PYRROLOPYRIDINE-DERIVATIVE AND PHARMACEUTICAL USE THEREOF<br/>[FR] DÉRIVÉ D'INDAZOLE ET PYRROLOPYRIDINE ET UTILISATION PHARMACEUTIQUE DE CELUI-CI申请人:DAINIPPON SUMITOMO PHARMA CO公开号:WO2012169649A1公开(公告)日:2012-12-13The present invention relates to a novel indazole- or pyrrolopyridine-derivative, represented by the formula (1) below, that has an agonistic action or a partial agonistic action against serotonin-4 receptor, and a pharmaceutical composition comprising the same. Formula (1) [wherein each substituent is as defined in claim 1]
-
[EN] PARG INHIBITORY COMPOUNDS<br/>[FR] COMPOSÉS INHIBITEURS DE PARG申请人:CANCER REC TECH LTD公开号:WO2016097749A1公开(公告)日:2016-06-23The present invention relates to compounds of formula I that function as inhibitors of PARG (Poly ADP-ribose glycohydrolase) enzyme activity: wherein R1a, R1b, R1c, R1d, R1e, W, X1, X2, X3, X4, X5, X6, X7, c are each as defined herein. The present invention also relates to processes for the preparation of these compounds, to pharmaceutical compositions comprising them, and to their use in the treatment of proliferative disorders, such as cancer, as well as other diseases or conditions in which PARG activity is implicated.
-
Potency and pharmacokinetics of GS-441524 derivatives against SARS-CoV-2作者:Daibao Wei、Tianwen Hu、Yumin Zhang、Wei Zheng、Haitao Xue、Jingshan Shen、Yuanchao Xie、Haji A. AisaDOI:10.1016/j.bmc.2021.116364日期:2021.9including five isobutyryl esters, two l-valine esters, and one carbamate. Among the new nucleosides, only the 7-fluoro analog 3c had moderate anti-SARS-CoV-2 activity, and its phosphoramidate prodrug 7 exhibited reduced activity in Vero E6 cells. As for the prodrugs, the 3′-isobutyryl ester 5a, the 5′-isobutyryl ester 5c, and the tri-isobutyryl ester 5g hydrobromide showed excellent oral bioavailabilities瑞德西韦的核苷代谢物 GS-441524 显示出有效的抗 SARS-CoV-2 功效,并且正在临床评估作为 COVID-19 的口服抗病毒治疗方法。然而,这种核苷在非人灵长类动物中的口服生物利用度较差,这可能会影响其治疗效果。在此,我们报道了多种在碱基或糖部分上进行修饰的 GS-441524 类似物,以及一些前药形式,包括五种异丁酯、两种L-缬氨酸酯和一种氨基甲酸酯。在新核苷中,只有7-氟类似物3c具有中等抗SARS-CoV-2活性,其氨基磷酸酯前药7在Vero E6细胞中表现出降低的活性。至于前药,3'-异丁酯5a 、5'-异丁酯5c和三异丁酯5g氢溴酸盐在小鼠中表现出优异的口服生物利用度(F 分别为 71.6%、86.6% 和 98.7%)。这为 GS-441524 的药代动力学优化提供了很好的见解。
-
Analogs of indole-3-carbinol metabolites as chemotherapeutic and chemopreventive agents申请人:——公开号:US20040043965A1公开(公告)日:2004-03-04Novel compounds useful as chemotherapeutic and chemopreventive agents are provided. The compounds are analogs of indole-3-carbinol metabolites wherein the structures and substituents of the compounds are selected to enhance the compounds' overall efficacy, particularly with respect to therapeutic activity, oral bioavailability, long-term safety, patient tolerability, and therapeutic window. The compounds are useful not only in treatment of cancer but also in prevention of cancer. One preferred class of the novel compounds have the structure of formula (I) 1 wherein R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , R 9 , R 10 , R 11 , and R 12 are defined herein. Pharmaceutical compositions are provided as well, as are methods of synthesis and use.
表征谱图
-
氢谱1HNMR
-
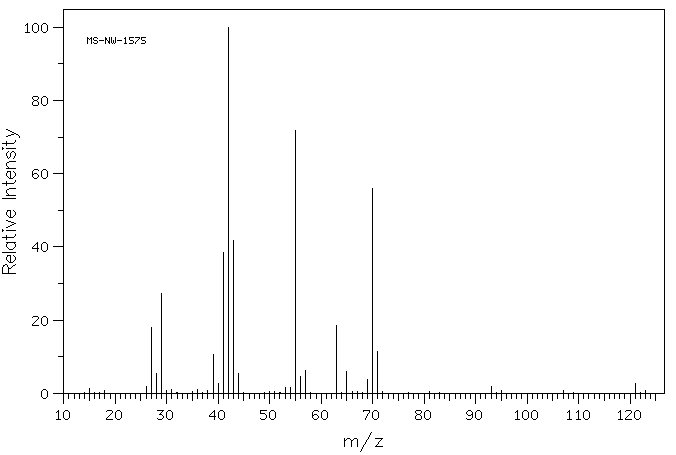
质谱MS
-
碳谱13CNMR
-
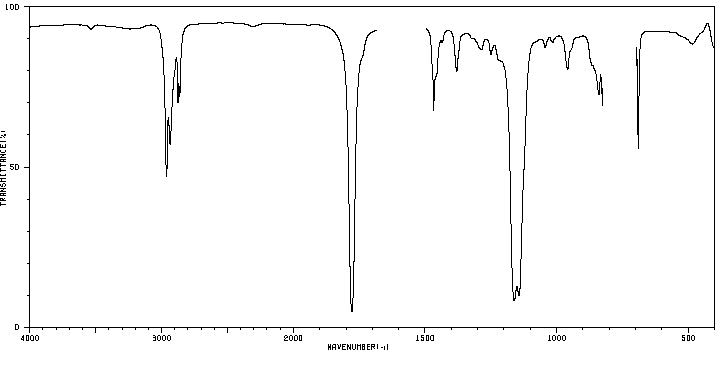
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-4-[(甲基氨基甲酰)氨基]环己烷羧酸
顺式-3-己烯醇碳酸甲酯
镏碳酸盐二水
镍,[碳酸(2-)-κO]-
镁(1-甲基-3-氧代-丁-1-烯基)碳酸氢酯
锌氮烷碳酸盐
锆碳酸盐氧化物
锂(1-羧基环丙基)锂
铵铜碳酸盐
铯碳酸氢钠
铝镁加
铝镁加
铝碳酸镁
铝碳酸镁
钠脲氯酸盐
钠甲基碳酸酯
钙钠碳酸氢盐氟化物
钙四镁钠碳酸氢盐三碳酸盐四氢氧化物
钐(+3)阳离子碳酸酯
重质碳酸镁
重碳酸钠-13C
酸氧(-2)阴离子铅杂亚酸碳
酮羧酸
邻苯二甲酸氢壬酯
过氧碳酸钠
过氧碳酸二钠盐
过氧碳酸,O,O'-1,6-亚己基-OO,OO'-二叔丁基酯
过氧化脲素
过氧化二碳酸双十四酯
过氧化二碳酸双十六酯
过氧化二碳酸二硬脂酰酯
过氧化二碳酸二环己酯
过氧化二碳酸二正丁酯
过氧化二碳酸二异丙酯
过氧化二碳酸二仲丁酯
过氧化二碳酸二乙酯
过氧化二碳酸二-3-甲氧基丁酯
过氧化二碳酸二(2-乙基己)酯
过氧化(2-乙基己基)碳酸叔戊酯
过氧二碳酸二十三烷酯
过氧二碳酸二丙基酯
达比加群酯杂质41
达比加群酯杂质22
达比加群杂质36
达比加群杂质19
辛酰脲
辛基辛氧基甲基碳酸酯
辛基脲
轻质碳酸镁
起始原料2杂质B