2-庚炔-1-醇 | 1002-36-4
中文名称
2-庚炔-1-醇
中文别名
2-庚烯-1-醇
英文名称
hep-2-yn-1-ol
英文别名
hept-2-yn-1-ol;2-heptyn-1-ol
CAS
1002-36-4
化学式
C7H12O
mdl
MFCD00039541
分子量
112.172
InChiKey
OGDYROFIIXDTQK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-35.25°C (estimate)
-
沸点:89-90 °C/16 mmHg (lit.)
-
密度:0.880 g/mL at 25 °C (lit.)
-
闪点:172 °F
-
稳定性/保质期:
在常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
安全说明:S26,S37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:2905290000
-
危险品运输编号:1987
-
危险标志:GHS08
-
危险性描述:H334
-
危险性防范说明:P261,P342 + P311
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
1.1 产品标识符
: 2-Heptyn-1-ol
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
呼吸敏化作用 (类别1)
急性的水体毒性 (类别2)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 危险
危险申明
H227 可燃液体
H334 吸入可能导致过敏或哮喘病症状或呼吸困难。
H401 对水生生物有毒。
警告申明
预防
P210 远离热源/火花/明火/热表面。- 禁止吸烟。
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P273 避免释放到环境中。
P280 戴防护手套/ 穿防护服/ 戴防护眼罩/ 戴防护面具。
P285 如通风不足,须戴呼吸防护面罩。
措施
P304 + P341 如误吸入:如呼吸困难,将受害人转移到空气新鲜处,保持呼吸舒适的休
息姿势。
P342 + P311 如有呼吸系统病症:呼叫解毒中心或医生。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P235 存放在通风良好的地方。保持低温。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H12O
分子式
: 112.17 g/mol
分子量
成分 浓度
2-Heptyn-1-ol
-
化学文摘编号(CAS No.) 1002-36-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 移去所有火源。
将人员撤离到安全区域。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境预防措施
在确保安全的条件下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
防止排放到周围环境中。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取防静电生成的措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
89 - 90 °C 在 21 hPa - lit.
g) 闪点
78 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
0.88 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
辛醇--水的分配系数的对数值: 1.534
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
热,火焰和火花。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
长期或反复接触导致个别人过敏反应
吸入可引起过敏。
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
对水生生物有毒。
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 2-Heptyn-1-ol
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
易燃液体 (类别4)
呼吸敏化作用 (类别1)
急性的水体毒性 (类别2)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 危险
危险申明
H227 可燃液体
H334 吸入可能导致过敏或哮喘病症状或呼吸困难。
H401 对水生生物有毒。
警告申明
预防
P210 远离热源/火花/明火/热表面。- 禁止吸烟。
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P273 避免释放到环境中。
P280 戴防护手套/ 穿防护服/ 戴防护眼罩/ 戴防护面具。
P285 如通风不足,须戴呼吸防护面罩。
措施
P304 + P341 如误吸入:如呼吸困难,将受害人转移到空气新鲜处,保持呼吸舒适的休
息姿势。
P342 + P311 如有呼吸系统病症:呼叫解毒中心或医生。
P370 + P378 火灾时: 用干的砂子,干的化学品或耐醇性的泡沫来灭火。
储存
P403 + P235 存放在通风良好的地方。保持低温。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H12O
分子式
: 112.17 g/mol
分子量
成分 浓度
2-Heptyn-1-ol
-
化学文摘编号(CAS No.) 1002-36-4
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
禁止催吐。 切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
小(起始)火时,使用媒介物如“乙醇”泡沫、干化学品或二氧化碳。大火时,尽可能使用水灭火。使用大量(
洪水般的)水以喷雾状应用;水柱可能是无效的。用大量水降温所有受影响的容器。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
水喷雾可用来冷却未打开的容器。
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 移去所有火源。
将人员撤离到安全区域。 防范蒸汽积累达到可爆炸的浓度,蒸汽能在低洼处积聚。
6.2 环境预防措施
在确保安全的条件下,采取措施防止进一步的泄漏或溢出。 不要让产物进入下水道。
防止排放到周围环境中。
6.3 抑制和清除溢出物的方法和材料
用防电真空清洁器或湿的刷子将溢出物收集起来并放置到容器中去,根据当地规定处理(见第13部分)。
存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止吸入蒸汽和烟雾。
切勿靠近火源。-严禁烟火。采取防静电生成的措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 起始沸点和沸程
89 - 90 °C 在 21 hPa - lit.
g) 闪点
78 °C - 闭杯
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
0.88 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
辛醇--水的分配系数的对数值: 1.534
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
热,火焰和火花。
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
长期或反复接触导致个别人过敏反应
吸入可引起过敏。
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
对水生生物有毒。
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
此易爆炸产品可以在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:2-庚炔-1-醇 在 吡啶 、 sodium dihydrogenphosphate 、 lithium aluminium tetrahydride 、 硫酸 、 sodium hydride 、 lithium chloride 作用下, 以 四氢呋喃 、 二氯甲烷 、 丙酮 为溶剂, 反应 51.5h, 生成 (2S, 3R)-(-)-1,2-epoxy-3-heptanol参考文献:名称:卤代烷基环氧化物的酶促对映聚合转化摘要:使用整个细菌细胞的环氧水解酶活性对 2,3-二取代外消旋-顺-和外消旋-反-卤代烷基环氧化物 1a-8a 进行生物催化水解,得到相应的邻位二醇 1b-8b 作为中间体;这些(自发地)经历闭环以通过酶触发的级联反应产生环状产物 1c-6c。特别是,顺式构型的底物(1a、3a、5a、7a)以对映收敛方式转化,导致从外消旋体中形成 100% des 和高达 92% ees 的单一立体异构产物。DOI:10.1002/1099-0690(200112)2001:23<4537::aid-ejoc4537>3.0.co;2-e
-
作为产物:描述:参考文献:名称:Shostakovskii,M.F. et al., Journal of Organic Chemistry USSR (English Translation), 1969, vol. 5, p. 204 - 207摘要:DOI:
文献信息
-
Acetylenic &agr;-amino acid-based sulfonamide hydroxamic acid tace inhibitors申请人:American Cyanamid Company公开号:US06225311B1公开(公告)日:2001-05-01Compounds of the formula: are useful in treating disease conditions mediated by TNF-&agr;, such as rheumatoid arthritis, osteoarthritis, sepsis, AIDS, ulcerative colitis, multiple sclerosis, Crohn's disease and degenerative cartilage loss.该式化合物在治疗由TNF-α介导的疾病条件中很有用,如类风湿性关节炎、骨关节炎、败血症、艾滋病、溃疡性结肠炎、多发性硬化症、克罗恩病和软骨退行性损失。
-
Electrotelluration: A New Approach to Tri- and Tetrasubstituted Alkenes作者:Joseph P. Marino、Hanh Nho NguyenDOI:10.1021/jo0110146日期:2002.9.1described in which a Michael addition of an alkyl or aryl tellurolate anion occurs onto an activated alkyne with subsequent trapping of a vinyl anion with electrophiles (aldehydes and ketones) other than a proton. This process provides an efficient regio- and stereospecific route to tri- and tetrasubstituted alkenes. Methodologically significant examples of this chemistry were studied in which aryl and alkyl
-
Synthesis of substituted quinolines by the electrophilic cyclization of n-(2-alkynyl)anilines作者:Xiaoxia Zhang、Tuanli Yao、Marino A. Campo、Richard C. LarockDOI:10.1016/j.tet.2009.12.012日期:2010.2A wide variety of substituted quinolines are readily synthesized under mild reaction conditions by the 6-endo-dig electrophilic cyclization of N-(2-alkynyl)anilines by ICl, I2, Br2, PhSeBr, and p-O2NC6H4SCl. The reaction affords 3-halogen-, selenium- and sulfur-containing quinolines in moderate to good yields in the presence of various functional groups. Analogous quinolines bearing a hydrogen in the
-
Rhodium(I)‐Catalyzed Cycloisomerization of Homopropargylallene‐Alkynes through C(sp <sup>3</sup> )−C(sp) Bond Activation作者:Yasuaki Kawaguchi、Kenya Yabushita、Chisato MukaiDOI:10.1002/anie.201713096日期:2018.4.16Upon exposure to a catalytic amount of [RhCl(CO)2]2 in 1,4‐dioxane, homopropargylallene‐alkynes underwent a novel cycloisomerization accompanied by the migration of the alkyne moiety of the homopropargyl functional group to produce six/five/five tricyclic compounds in good yields. A plausible mechanism was proposed on the basis of an experiment with 13C‐labeled substrate. The resulting tricyclic derivatives
-
Asymmetric Synthesis of 3-Allyloxindoles and 3-Allenyloxindoles by Scandium(III)-Catalyzed Claisen Rearrangement Reactions作者:Zeng-Wei Lai、Chuan Liu、Hongbin Sun、Shu-Li YouDOI:10.1002/cjoc.201700486日期:2017.10Scandium‐catalyzed asymmetric Claisen rearrangement reactions of 2‐allyloxyindoles and 2‐propargyloxyindoles provide a novel approach to diverse 3‐allyloxindoles and 3‐allenyloxindoles in excellent yields (up to 99%) and enantioselectivity (up to 99% ee) under mild reaction conditions. The scandium catalyst was derived from Sc(OTf)3 and Pybox ligand.
表征谱图
-
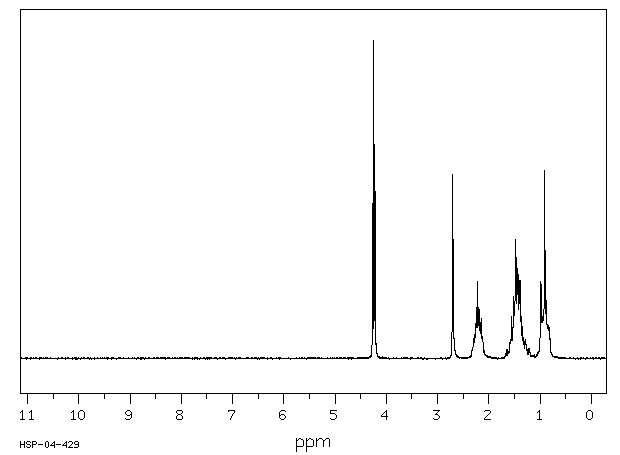
氢谱1HNMR
-
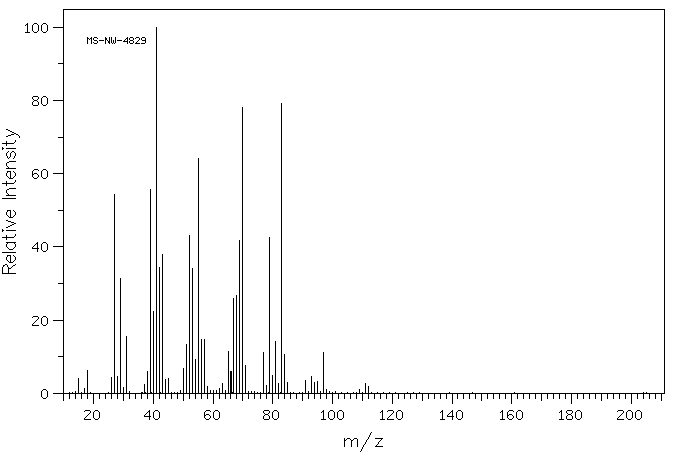
质谱MS
-
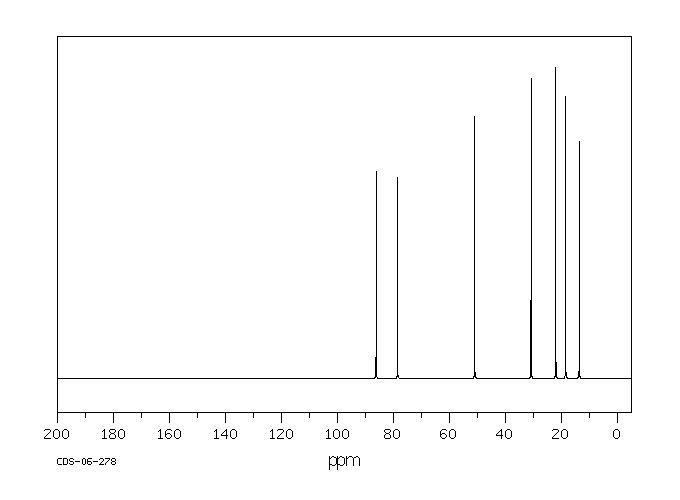
碳谱13CNMR
-
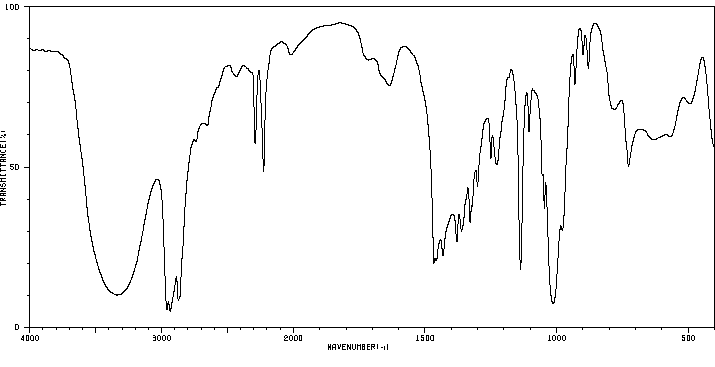
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯