2-庚炔-4-酮 | 71932-98-4
中文名称
2-庚炔-4-酮
中文别名
——
英文名称
hept-2-yn-4-one
英文别名
2-heptyne-4-one;Hept-2-in-4-on
CAS
71932-98-4
化学式
C7H10O
mdl
——
分子量
110.156
InChiKey
NGDLDSJVXIMWCH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:8
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.57
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
SDS
反应信息
-
作为反应物:参考文献:名称:Total Synthesis of Amphidinolide J摘要:The marine natural product amphidinolide J has been synthesized according to a convergent strategy. The key steps of this synthesis include a B-alkyl Suzuki-Miyaura coupling and the addition of an alkynyllithium reagent to a Weinreb amide to build the C4-C5 and C12-C13 bonds, respectively, and a Yamaguchi macrolactonization.DOI:10.1021/ol801708x
-
作为产物:参考文献:名称:Martin, Annales de Chimie (Cachan, France), 1959, vol. <13>1, p. 541,561摘要:DOI:
文献信息
-
Efficient methods for the preparation of acetylenic ketones作者:H.D. Verkruijsse、Y.A. Heus-Kloos、L. BrandsmaDOI:10.1016/0022-328x(88)80002-0日期:1988.1A number of acetylenic ketones RCCC(O)R′ have been obtained in good yields from lithiated acetylenes RCCLi and acetic anhydride, N,N-dimethylacetamide, or N,N-dimethylbenzamide. The most convenient and general method consists of treating alkynylzinc chlorides with acid halides R′C(O)Cl. Benzoyl chloride (R′ = Ph), acryloyl chloride (R′ = CH2CH), and butynoyl chloride (R′ = C2H5CC) react only
-
Metal Cation-Exchanged Montmorillonite (M<sup><i>n</i>+</sup>-Mont)-Catalyzed Friedel–Crafts Acylation of 1-Methyl-1-cyclohexene and 1-Trimethylsilyl-1-alkynes作者:Takahiro Nishimura、Seiji Ohtaka、Keiji Hashimoto、Takayoshi Yamauchi、Takuji Hasegawa、Kaori Imanaka、Jun-ichi Tateiwa、Hiroshi Takeuchi、Sakae UemuraDOI:10.1246/bcsj.77.1765日期:2004.9The acylation of 1-methyl-1-cyclohexene and 1-trimethylsilyl-1-alkynes with acyl chlorides has been investigated in the presence of a variety of metal cation-exchanged montmorillonites (abbreviated as Mn+-monts), where the catalysts are recyclable for several times after simple washing.
-
A Novel Cu-Assisted Cycloisomerization of Alkynyl Imines: Efficient Synthesis of Pyrroles and Pyrrole-Containing Heterocycles作者:Alexander V. Kel'in、Anna W. Sromek、Vladimir GevorgyanDOI:10.1021/ja0058684日期:2001.3.1
-
Palladium(II)-Catalyzed Three-Component Coupling Reaction Initiated by Acetoxypalladation of Alkynes: An Efficient Route to γ,δ-Unsaturated Carbonyls作者:Ligang Zhao、Xiyan LuDOI:10.1021/ol026741h日期:2002.10.1[GRAPHICS]A divalent palladium-catalyzed coupling reaction of electron-deficient alkynes and acrolein or MVK (methyl vinyl ketone) was developed. The reaction provides an efficient method to synthesize gamma,delta-unsaturated carbonyls. A mechanism involving acetoxypalladation of alkyne, followed by insertion of alkene and protonolysis of the C-Pd bond, is proposed. The protonolysis of the carbon-palladium bond with the assistance of bidentate nitrogen containing ligands is the key step in this tandem reaction.
-
Moody, Christopher J.; Shah, Pritom, Journal of the Chemical Society. Perkin transactions I, 1988, p. 1407 - 1416作者:Moody, Christopher J.、Shah, PritomDOI:——日期:——
表征谱图
-
氢谱1HNMR
-
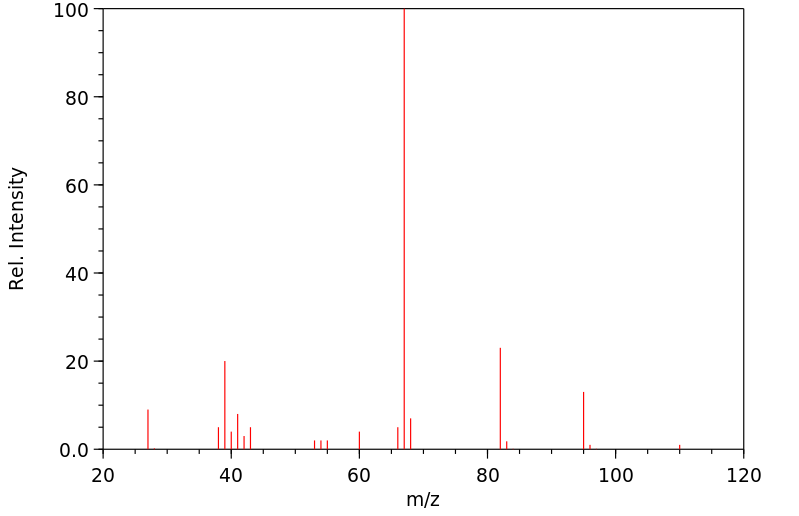
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷