1-甲基-5-巯基-1H-四氮唑 | 13183-79-4
中文名称
1-甲基-5-巯基-1H-四氮唑
中文别名
5-巯基-1-甲基四唑;5-巯基-1-甲基四唑(MMT);5-巯基-1-甲基四氮唑;甲巯四氮唑;1-甲基-5-巯基-1,2,3,4-四氮唑;1-甲基四氮唑-5-硫醇;1-甲基-1H-四氮唑-5-硫醇;甲基巯基四氮唑;1-甲基-5-巯基四氮唑;5-巯基-1-甲基四唑/甲硫四唑;1-甲基-巯基-1,2,3,4-四氮唑;甲硫四氮唑;1-甲基-5-巯基-1H-四唑
英文名称
1-methyl-5-mercaptotetrazole
英文别名
5-mercapto-1-methyltetrazole;1-methyl-1H-tetrazole-5-thiol;1-methyltetrazole-5-thiol;1-methyl-2H-tetrazole-5-thione
CAS
13183-79-4
化学式
C2H4N4S
mdl
MFCD00037317
分子量
116.147
InChiKey
XOHZHMUQBFJTNH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:125-128 °C (lit.)
-
沸点:111.6±23.0 °C(Predicted)
-
密度:1.420 (estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶)、水(微溶)
-
稳定性/保质期:
在常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.3
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:72.1
-
氢给体数:1
-
氢受体数:3
安全信息
-
TSCA:Yes
-
安全说明:S26,S37/39
-
WGK Germany:3
-
海关编码:2933990090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险品运输编号:25kgs
-
RTECS号:XF7600000
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
包装等级:II
-
危险类别:4.1
-
储存条件:常温下应存于密闭容器中,并置于阴凉、通风且干燥处。
SDS
5-巯基-1-甲基四唑 修改号码:5
模块 1. 化学品
产品名称: 5-Mercapto-1-methyltetrazole
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃固体 第2级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃固体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
使用防爆的电气/通风/照明设备。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
5-巯基-1-甲基四唑 修改号码:5
模块 3. 成分/组成信息
化学名(中文名): 5-巯基-1-甲基四唑
百分比: >98.0%(LC)(T)
CAS编码: 13183-79-4
俗名: 1-Methyl-5-mercaptotetrazole , 1-Methyltetrazole-5-thiol
分子式: C2H4N4S
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
5-巯基-1-甲基四唑 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 126°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
自燃温度: 256°C
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 火花, 明火, 静电, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:400 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: XF7600000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
5-巯基-1-甲基四唑 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 第1项 易燃固体。
UN编号: 1325
正式运输名称: 可燃固体, 有机的, 不另作详细说明.
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 5-Mercapto-1-methyltetrazole
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害
易燃固体 第2级
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 易燃固体
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
使用防爆的电气/通风/照明设备。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
5-巯基-1-甲基四唑 修改号码:5
模块 3. 成分/组成信息
化学名(中文名): 5-巯基-1-甲基四唑
百分比: >98.0%(LC)(T)
CAS编码: 13183-79-4
俗名: 1-Methyl-5-mercaptotetrazole , 1-Methyltetrazole-5-thiol
分子式: C2H4N4S
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 一旦发生火警,有爆炸的危险。由于爆炸的危险,远距离灭火。
小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。切勿引起泄漏、溢出或分散。切勿产
生不必要的蒸气。远离热源/火花/明火/热表面。禁烟。采取措施防止静电积累。使用
防爆设备。避免冲击和摩擦。处理后彻底清洗双手和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
确保容器不会受到未测影响,比如跌落或脱落。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
5-巯基-1-甲基四唑 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点: 126°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 溶于
[其他溶剂] 无资料
自燃温度: 256°C
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 热、撞击、摩擦等条件下可能爆炸分解。
避免接触的条件: 热, 火花, 明火, 静电, 冲击, 摩擦
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: ipr-mus LD50:400 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: XF7600000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
5-巯基-1-甲基四唑 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门和专家。建议在可燃溶剂中溶解混合,然后在装有后燃和洗涤装置的化
学焚烧炉中慢慢焚烧。如果一次性焚烧大量物质,可能发生爆炸。废弃处置时请遵守国家、地区和当地的所有法规
。
模块 14. 运输信息
联合国分类: 第1项 易燃固体。
UN编号: 1325
正式运输名称: 可燃固体, 有机的, 不另作详细说明.
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-甲基-1H-四唑 1-methyl-1,2,3,4-tetrazole 16681-77-9 C2H4N4 84.0806 —— 1-Hydroxymethyl-4-methyl-1,4-dihydro-tetrazole-5-thione 169692-54-0 C3H6N4OS 146.173
反应信息
-
作为反应物:描述:参考文献:名称:Improved efficiency of CdS quantum dot sensitized solar cell with an organic redox couple and a polymer counter electrode摘要:DOI:10.1016/j.electacta.2014.06.072
-
作为产物:描述:参考文献:名称:通过药物再利用发现喹唑啉衍生物作为一类新的强效和体内有效的 LSD1 抑制剂摘要:组蛋白赖氨酸特异性去甲基化酶 1 (LSD1) 是一种重要的表观遗传调节剂,并以不同的方式参与恶性转化和肿瘤发病机制。因此,LSD1的抑制为癌症治疗提供了一个有吸引力的治疗靶点。基于药物再利用策略,我们筛选了我们内部针对 LSD1 的化学库,发现 EGFR 抑制剂厄洛替尼(一种 FDA 批准的肺癌药物)对 LSD1 的效力较低(IC 50 = 35.80 μM)。在此,我们报告了我们进一步的药物化学努力,以获得高度水溶性的厄洛替尼类似物5k (>100 mg/mL),其对 LSD1 的抑制活性显着增强 (IC 50 = 0.69 μM) 以及更高的特异性。在 MGC-803 细胞中,5k抑制 LSD1 的去甲基化,表明其对酶的细胞活性。此外,5k具有显着的抑制集落形成、抑制迁移和诱导MGC803细胞凋亡的能力。此外,在 MGC-803 异种移植小鼠模型中,5k治疗导致肿瘤大小分别在 40DOI:10.1016/j.ejmech.2021.113778
-
作为试剂:描述:胞苷 在 咪唑 、 ammonium hydroxide 、 四丁基氟化铵 、 1-甲基-5-巯基-1H-四氮唑 作用下, 以 四氢呋喃 、 吡啶 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 反应 29.34h, 生成参考文献:名称:Structure–activity relationship of phosmidosine: importance of the 7,8-dihydro-8-oxoadenosine residue for antitumor activity摘要:To study the structure-activity relationship of phosmidosine, a variety of phosmidosine derivatives 9a-g were synthesized by condensation of N-diisopropyl N'-(N-tritylprolyl)phosphorodiamidite 6 with appropriately protected nucleoside derivatives 7a-g. As the result, replacement of the 7,8-diliydro-8-oxoadenine base by adenine and 6-N-acetyladenine did not affect the antitumor activity. However, phosmidosine derivatives containing uracil, cytosine, and guanine in place of the 7,8-dibydro-8-oxoadenine base did not show significant activity. A plausible explanation for the selective expression of phosmidosine compared with that of phosmidosine analogs having other amino acids in place of proline is also discussed. These results suggest that phosmidosine serves as an inhibitor of prolyl adenosine 5'-phosphate (prolyl-AMP) to inhibit the peptide synthesis in cancer-related cells. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2004.07.021
文献信息
-
Substituent-dependent coordination modes of 1-methyl-5-R-tetrazoles in their cupric chloride complexes作者:Sergei V. Voitekhovich、Alexander S. Lyakhov、Vadim E. Matulis、Ludmila S. Ivashkevich、Oleg A. IvashkevichDOI:10.1016/j.poly.2019.01.009日期:2019.4Abstract A series of cupric chloride complexes with 1-methyl-5-R-tetrazole ligands, where R = NH2, t-Bu, Ph, MeS, CH CH2, MeSO2, were synthesized by interaction of CuCl2·2H2O with the above ligands LNH2, LtBu, LPh, LMeS, LVin, and LMeSO2, respectively. The obtained complexes [Cu(LNH2)3Cl2]·H2O (1), [Cu(LtBu)2Cl2] (2), [Cu(LPh)2Cl2] (3), [Cu(LMeS)2Cl2] (4), [Cu(LVin)Cl2]n (5), and [Cu(LMeSO2)(H2O)Cl2]n摘要通过CuCl2·2 与上述配体LNH2的相互作用,合成了一系列具有1-甲基-5-R-四唑配体的氯化铜配合物,其中R = NH2,t-Bu,Ph,MeS,CH CH2,MeSO2 ,LtBu,LPh,LMeS,LVin和LMeSO2。获得的配合物[Cu(LNH2)3Cl2]·H2O(1),[Cu(LtBu)2Cl2](2),[Cu(LPh)2Cl2](3),[Cu(LMeS)2Cl2](4),[通过单晶X射线分析表征了Cu(LVin)Cl 2] n(5)和[Cu(LMeSO 2)(H 2 O)Cl 2] n(6)。观察到C5-取代基对四唑配体配位方式和配合物结构基序的影响。配合物1-4为单核,具有四唑环N4配位。配合物5呈现一维配位聚合物,其形成是以两个相邻铜(II)阳离子之间的三桥(双氯桥和四唑环N3,N4-桥)为代价的。在6 作为一维配位聚合物,配位链由交替的Cu(LMeSO2)
-
一种抗生素改进型的盐酸头孢甲肟合成工艺申请人:重庆化工职业学院公开号:CN112321609A公开(公告)日:2021-02-05本发明公开了一种抗生素改进型的盐酸头孢甲肟合成工艺,一种抗生素改进型的盐酸头孢甲肟合成工艺,包括如下步骤:步骤一:1‑甲基‑5‑巯基‑1H‑四氮唑的制备;步骤二:2‑(2‑氨基‑4‑嚦唑基)‑2(Z)‑甲氧亚胺基乙酸乙酯的制备;步骤三:2‑(2‑氨基‑4‑噻唑基)‑2(Z)‑甲氧亚胺基乙酸的制备;步骤四:2‑(2‑氨基‑4‑噻唑基)‑2(Z)‑甲氧亚胺基乙酸苯‑2‑并噻唑硫酯的制备;步骤五:7‑氨基‑3‑(1‑甲基‑1H‑四氮唑‑5‑硫甲基)头孢烷酸盐酸盐(7‑ACA‑MMT·HC1)的制备;步骤六:盐酸头孢甲肟的制备;本发明采用国产原料,具备合成路线原料廉价易得,降低了合成的成本,合成改进;改进后的工艺原料成本低廉,操作简单,适合工业生产的优点。
-
The development of a complementary pathway for the synthesis of aliskiren作者:Le-Le Li、Jin-Ying Ding、Lian-Xun Gao、Fu-She HanDOI:10.1039/c4ob01963f日期:——The synthesis of aliskiren (1), a recently marketed drug for the treatment of hypertension, is presented. The focus of our synthetic effort is to develop an efficient pathway for the synthesis of (2S,7R,E)-2-isopropyl-7-(4-methoxy-3-(3-methoxypropoxy) benzyl)-N,N,8-trimethylnon-4-enamide (2a), which has been used as the advanced intermediate toward aliskiren. After an extensive investigation of three介绍了阿利吉仑(1)的合成,阿利吉仑(1)是一种最近上市的用于治疗高血压的药物。我们合成工作的重点是开发合成(2S,7R,E)-2-异丙基-7-(4-甲氧基-3-(3-甲氧基丙氧基)苄基)-N,N,8的有效途径-trimethylnon-4-enamide(2a),已被用作阿利吉仑的高级中间体。在对通过使用烯烃交叉复分解,Horner-Wadsworth-Emmons(HWE)和Julia型烯化反应在2a中构建E-烯烃官能度的三种不同策略进行了广泛研究之后,我们建立了合成的新方案在产率(约33%)以及非对映和E / Z选择性方面,显着提高了2a的总效率。关键的转化是埃文斯手性辅助不对称烯丙基化,用于合成合适的手性中间体,其对映体纯度高于97%ee,修饰的Julia-Kocienski烯化用于E-2a的高选择性构建,最高可达13.6来自手性中间体的E / Z比为1:1。因此,结果为阿利吉仑的合成提供了一个有吸引力的选择。
-
1-Substituted-5-[(3,5-dinitrobenzyl)sulfanyl]-1H-tetrazoles and their isosteric analogs: A new class of selective antitubercular agents active against drug-susceptible and multidrug-resistant mycobacteria作者:Galina Karabanovich、Jaroslav Roh、Tomáš Smutný、Jan Němeček、Petr Vicherek、Jiřina Stolaříková、Marcela Vejsová、Ida Dufková、Kateřina Vávrová、Petr Pávek、Věra Klimešová、Alexandr HrabálekDOI:10.1016/j.ejmech.2014.05.069日期:2014.7In this work, a new class of highly potent antituberculosis agents, 1-substituted-5-[(3,5-dinitrobenzyl)sulfanyl]-1H-tetrazoles and their oxa and selanyl analogs, is described. The minimal inhibitory concentration (MIC) values reached 1 μM (0.36–0.44 μg/mL) against Mycobacterium tuberculosis CNCTC My 331/88 and 0.25–1 μM against six multidrug-resistant clinically isolated strains of M. tuberculosis在这项工作中,描述了一类新型的高效抗结核药,即1-取代的5-[(3,5-二硝基苄基)硫烷基] -1 H-四唑及其氧杂和硒基类似物。最小抑菌浓度(MIC)值达到了1μM(0.36-0.44微克/毫升)对结核分枝杆菌CNCTC我八十八分之三百三十一和0.25-1μM针对六个多药耐药性临床分离菌株的结核分枝杆菌。这些化合物的抗分枝杆菌作用具有高度特异性,因为它们对所研究的所有八种细菌菌株和八种真菌菌株均无效。此外,这些化合物在四种哺乳动物细胞系中均表现出较低的体外毒性(IC 50 > 30μM)。我们还检查了化合物的结构-活性关系,特别是硝基基团的数量和位置,四唑和苄基部分之间的连接基以及四唑本身对抗分枝杆菌活性的影响。在不对分枝杆菌活性产生负面影响的情况下,四唑上取代基R 1的相对较高的变异性可进一步优化毒性,并进一步优化1-取代的5-[(3,5-二硝基苄基)硫烷基]-的ADME性质。 1 H-四唑化合物。
-
[EN] 4-PIPERAZINYL-PYRIMIDINE COMPOUNDS SUITABLE FOR TREATING DISORDERS THAT RESPOND TO MODULATION OF THE DOPAMINE D3 RECEPTOR<br/>[FR] COMPOSÉS DE 4-PIPÉRAZINYLPYRIMIDINE CONVENANT POUR TRAITER DES TROUBLES QUI RÉPONDENT À UNE MODULATION DU RÉCEPTEUR D3 DE LA DOPAMINE申请人:ABBOTT GMBH & CO KG公开号:WO2006015842A1公开(公告)日:2006-02-16The present invention relates to novel 4-piperazinylpyrimidine compounds. The compounds possess valuable therapeutic properties and are suitable, in particular, for treating diseases that respond to modulation of the dopamine D3 receptor. The 4 piperzinylpyrimidine compounds have the general formula (I), wherein Ar, X, A, R1 and R1a are as defined in the claims.
表征谱图
-
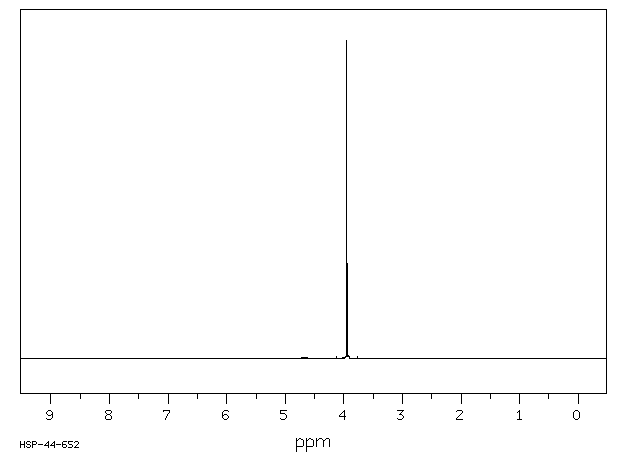
氢谱1HNMR
-
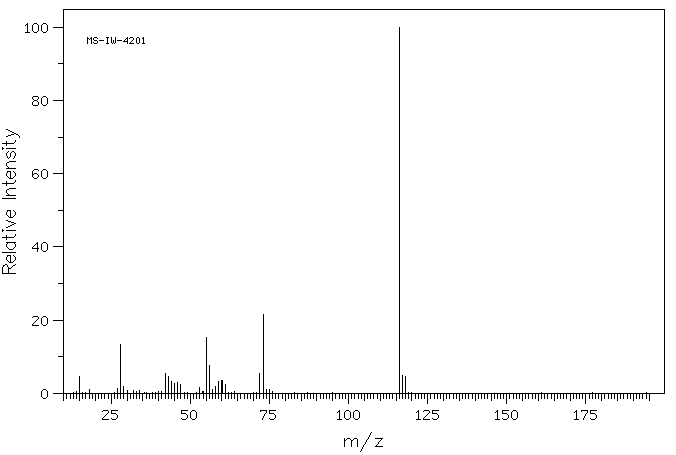
质谱MS
-
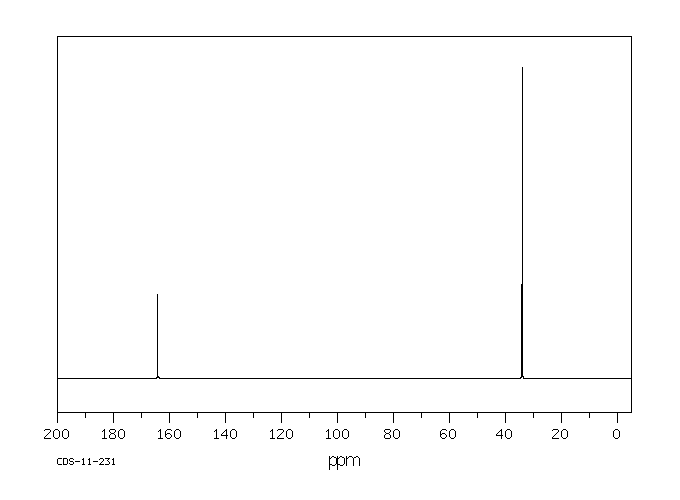
碳谱13CNMR
-
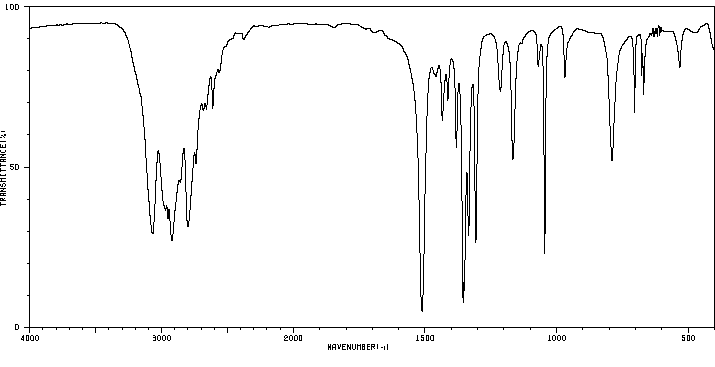
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(SP-4-1)-二氯双(1-苯基-1H-咪唑-κN3)-钯
(5aS,6R,9S,9aR)-5a,6,7,8,9,9a-六氢-6,11,11-三甲基-2-(2,3,4,5,6-五氟苯基)-6,9-甲基-4H-[1,2,4]三唑[3,4-c][1,4]苯并恶嗪四氟硼酸酯
(5-氨基-1,3,4-噻二唑-2-基)甲醇
齐墩果-2,12-二烯[2,3-d]异恶唑-28-酸
黄曲霉毒素H1
高效液相卡套柱
非昔硝唑
非布索坦杂质Z19
非布索坦杂质T
非布索坦杂质K
非布索坦杂质E
非布索坦杂质D
非布索坦杂质67
非布索坦杂质65
非布索坦杂质64
非布索坦杂质61
非布索坦代谢物67M-4
非布索坦代谢物67M-2
非布索坦代谢物 67M-1
非布索坦-D9
非布索坦
非唑拉明
雷非那酮-d7
雷西那德杂质2
雷西纳德杂质L
雷西纳德杂质H
雷西纳德杂质B
雷西纳德
雷西奈德杂质
阿西司特
阿莫奈韦
阿考替胺杂质9
阿米苯唑
阿米特罗13C2,15N2
阿瑞匹坦杂质
阿格列扎
阿扎司特
阿尔吡登
阿塔鲁伦中间体
阿培利司N-1
阿哌沙班杂质26
阿哌沙班杂质15
阿可替尼
阿作莫兰
阿佐塞米
镁(2+)(Z)-4'-羟基-3'-甲氧基肉桂酸酯
锌1,2-二甲基咪唑二氯化物
锌(II)(苯甲醇)(四苯基卟啉)
锌(II)(正丁醇)(四苯基卟啉)
锌(II)(异丁醇)(四苯基卟啉)