4,4'-双(4-氨基苯氧基)二苯砜 | 13080-89-2
中文名称
4,4'-双(4-氨基苯氧基)二苯砜
中文别名
4,4’-双(4-氨基苯氧基)二苯砜;二[4-(4-氨基苯氧基)苯基]砜;双[4-(4-氨基苯氧基)苯基]砜;4,4"-双(4-氨基苯氧基)二苯砜
英文名称
bis[4-(4-aminophenoxy)phenyl]sulfone
英文别名
BAPS;Benzenamine, 4,4'-[sulfonylbis(4,1-phenyleneoxy)]bis-;4-[4-[4-(4-aminophenoxy)phenyl]sulfonylphenoxy]aniline
CAS
13080-89-2
化学式
C24H20N2O4S
mdl
MFCD00039155
分子量
432.5
InChiKey
UTDAGHZGKXPRQI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:195 °C
-
沸点:655.3±55.0 °C(Predicted)
-
密度:1.2136 (rough estimate)
-
溶解度:溶于丙酮
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:31
-
可旋转键数:6
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:113
-
氢给体数:2
-
氢受体数:6
安全信息
-
危险等级:6.1
-
危险品运输编号:UN 2811
-
RTECS号:BY8955000
-
海关编码:2922299090
-
包装等级:III
-
危险类别:6.1
-
储存条件:2-8°C
SDS
Bis[4-(4-aminophenoxy)phenyl] Sulfone Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Bis[4-(4-aminophenoxy)phenyl] Sulfone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 3
Acute toxicity (Oral)
Acute toxicity (Dermal) Category 3
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Toxic in contact with skin and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
Do not eat, drink or smoke when using this product.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Rinse
mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Remove/Take off immediately all contaminated clothing.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Bis[4-(4-aminophenoxy)phenyl] Sulfone
Percent: >98.0%(LC)(T)
CAS Number: 13080-89-2
Chemical Formula: C24H20N2O4S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:195 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Acetone
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:220 mg/kg
skn-rbt LD50:560 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: BY8955000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 6.1: Toxic substance.
UN-No: 2811
Proper shipping name: Toxic solid, organic, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: Bis[4-(4-aminophenoxy)phenyl] Sulfone
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 3
Acute toxicity (Oral)
Acute toxicity (Dermal) Category 3
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement Toxic in contact with skin and if swallowed
Causes skin irritation
Causes serious eye irritation
Precautionary statements
Do not eat, drink or smoke when using this product.
[Prevention]
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. Rinse
mouth.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Remove/Take off immediately all contaminated clothing.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): Bis[4-(4-aminophenoxy)phenyl] Sulfone
Percent: >98.0%(LC)(T)
CAS Number: 13080-89-2
Chemical Formula: C24H20N2O4S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust. Also install safety shower and eye bath.
Engineering controls:
Personal protective equipment
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Hand protection: Impervious gloves.
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: crystal - powder
Color: Very pale yellow - Yellow red
Odor: No data available
pH: No data available
Melting point/freezing point:195 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
Upper: No data available
Density: No data available
Solubility: Soluble in : Acetone
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Stability:
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: orl-rat LD50:220 mg/kg
skn-rbt LD50:560 mg/kg
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: BY8955000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobillity in soil
log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Bis[4-(4-aminophenoxy)phenyl] Sulfone
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 6.1: Toxic substance.
UN-No: 2811
Proper shipping name: Toxic solid, organic, n.o.s.
III
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— Benzene, 1,1'-sulfonylbis[4-(4-nitrophenoxy)- 27594-94-1 C24H16N2O8S 492.466 2,2'-磺酰基二苯酚 4,4'-sulfonediphenol 80-09-1 C12H10O4S 250.275 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,1'-(((Sulfonylbis(4,1-phenylene))bis(oxy))bis(4,1-phenylene))bis(1H-pyrrole-2,5-dione) —— C32H20N2O8S 592.6
反应信息
-
作为反应物:参考文献:名称:KANAYAMA, KAORU;OGUMA, JOSINOBU摘要:DOI:
-
作为产物:描述:参考文献:名称:Process for the preparation of 4,4'-bis(4-aminophenoxy)-diphenyl sulfone摘要:高纯度4,4'-双(4-氨基苯氧基)二苯砜是通过使用氨基酚、碱金属氢氧化物和4,4'-二卤二苯砜,按照碱金属氢氧化物:氨基酚在1.0-1.1:1.0范围内的摩尔比和氨基酚:4,4'-二卤二苯砜在2.005-2.05:1.0范围内的摩尔比,以及使用二烷胺或N-烷基内酰胺作为溶剂制备而成。获得了99.0%纯度的原始产品(无再结晶),熔点高于195摄氏度。公开号:US05118848A1
文献信息
-
Diamine Compound Having Phosphorylcholine Group, Polymer Thereof, and Process for Producing the Polymer申请人:Nagase Yu公开号:US20100036081A1公开(公告)日:2010-02-11Highly polymerizable diamine compounds having a phosphorylcholine group are disclosed. High-molecular weight polymers are obtained from the highly polymerizable diamine compound having a phosphorylcholine group as a monomer, and the polymers have improved mechanical strength, water resistance and heat resistance while maintaining excellent biocompatibility and processability of MPC polymers. Processes for producing the polymers are disclosed. The diamine compounds having a phosphorylcholine group are represented by Formula (I). The polymers contain at least 1 mol % of a specific structural unit with a phosphorylcholine group represented by Formula (II) and have a number average molecular weight of not less than 5,000. In the processes, the diamine compound is used as a monomer.
-
Synthesis and characterization of novel bio-based benzoxazines from eugenol作者:P. Thirukumaran、A. Shakila、Sarojadevi MuthusamyDOI:10.1039/c3ra46582a日期:——Polybenzoxazines are phenolic-like materials that possess dimensional and thermal stability, and they release no by-products during their polymerization. In this study, a new class of benzoxazine-containing monomers has been prepared from renewable resource (eugenol, a natural phenol obtained from clove oil), paraformaldehyde and various aromatic diamines. The structures of the monomers were supported by Fourier Transform Infrared (FT-IR), 1H-NMR and 13C-NMR spectral analysis, proving the existence of the benzoxazine ring containing eugenol moiety in its molecular structure. The monomers were polymerized/cured via ring-opening polymerization by heating as indicated by FT-IR and Differential Scanning Calorimetry (DSC). This is confirmed by the disappearance of the peaks due to oxazine ring (942 cm−1). The exothermic peak associated with curing was observed from 170 °C to 250 °C. The thermal and mechanical properties of the polybenzoxazines were investigated through thermogravimetric analysis (TGA) and dynamic mechanical analysis (DMA). The temperatures corresponding to 5% and 10% weight loss is from 240 to 295 °C and from 290 to 340 °C, respectively. The completely cured materials could achieve char yields up to 36.5% at 800 °C in nitrogen atmosphere. DMA revealed that the glass transition temperatures of PBz-SUL and PBz-PHE were higher than that of PBz-DDS and PBz-OXY.多苯并噁嗪是一种类似酚醛的材料,具有尺寸和热稳定性,并且在聚合过程中不释放副产物。在本研究中,从可再生资源(丁香油中获得的天然酚类化合物丁子香酚)、多聚甲醛和各种芳香二胺合成了一类新型的含苯并噁嗪单体。通过傅里叶变换红外光谱(FT-IR)、核磁共振(1H-NMR和13C-NMR)光谱分析验证了单体的结构,证实了其分子结构中存在含有丁子香酚部分的苯并噁嗪环。如FT-IR和差示扫描量热法(DSC)所示,单体通过加热开环聚合/固化。这一过程通过消失的噁嗪环特征峰(942 cm−1)得到证实。固化相关的放热峰在170 °C到250 °C之间被观察到。通过热重分析(TGA)和动态力学分析(DMA)研究了多苯并噁嗪的热性能和机械性能。对应于5%和10%质量损失的温度分别在240到295 °C和290到340 °C之间。在氮气气氛下,完全固化的材料在800 °C时可以达到高达36.5%的炭化产率。DMA显示,PBz-SUL和PBz-PHE的玻璃化转变温度高于PBz-DDS和PBz-OXY。
-
PHOTOREACTIVE COMPOUNDS申请人:Lincker Frederic公开号:US20140192305A1公开(公告)日:2014-07-10The present invention relates to photoreactive compounds that are particularly useful in materials for the alignment of liquid crystals.本发明涉及光反应性化合物,特别适用于液晶对准材料的制作。
-
[EN] PHOTOACTIVE MATERIALS<br/>[FR] MATERIAUX PHOTOACTIFS申请人:ROLIC AG公开号:WO2004013086A1公开(公告)日:2004-02-12Diamine compounds, which in particular are useful as precursors for the production of liquid crystal alignment layers, represented by general formula (I), wherein A1 represents an organic group of 1 to 40 carbon atoms; A2 represents a hydrogen atom or an organic group of 1 to 40 carbon atoms.二胺化合物,特别是作为液晶取向层生产的前体物而有用,由一般式(I)代表,其中A1代表1至40个碳原子的有机基团;A2代表氢原子或1至40个碳原子的有机基团。
-
Compound having silsesquioxane skeleton and its polymer申请人:Inagaki Jyun-ichi公开号:US20050009982A1公开(公告)日:2005-01-13The present invention relates to a compound represented by Formula (1) and a polymer obtained using the compound: wherein R 1 is phenyl which may have substituents, Q 1 is hydrogen, halogen, alkyl having 1 to 10 carbon atoms, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl or phenyl in which optional hydrogen may be replaced by halogen or alkyl having 1 to 5 carbon atoms, and Q 2 is a group represented by Formula (2) wherein the code < represents a bonding point with silicon, l, m, n and p are independently 0, 1, 2 or 3, A 1 to A 4 are independently a single bond, 1,4-cyclohexylene, 1,4-cyclohexenylene, a condensed ring group having 6 to 10 carbon atoms which is a divalent group, or 1,4-phenylene, Z 0 to Z 3 are independently a single bond, —CH═CR—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms, and Z 4 is a single bond, —CH═CH—, —C≡C—, —COO—, —OCO—, or alkylene having 1 to 20 carbon atoms. And Y 1 in Formula (1) is the group defined in Claim 1.本发明涉及一种由式(1)表示的化合物和使用该化合物获得的聚合物:其中R1是苯基,可能具有取代基;Q1是氢、卤素、具有1至10个碳原子的烷基、环丙基、环丁基、环戊基、环己基、环己烯基或苯基,其中可选的氢原子可被卤素或具有1至5个碳原子的烷基取代;Q2是由式(2)表示的基团,其中代码<表示与硅的连接点,l、m、n和p独立地为0、1、2或3,A1至A4独立地为单键、1,4-环己亚基、1,4-环己烯亚基、具有6至10个碳原子的缩合环基团,为二价基团,或1,4-苯亚基,Z0至Z3独立地为单键、—CH═CR—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基,Z4为单键、—CH═CH—、—C≡C—、—COO—、—OCO—或具有1至20个碳原子的烷基。式(1)中的Y1是权利要求1中定义的基团。
表征谱图
-
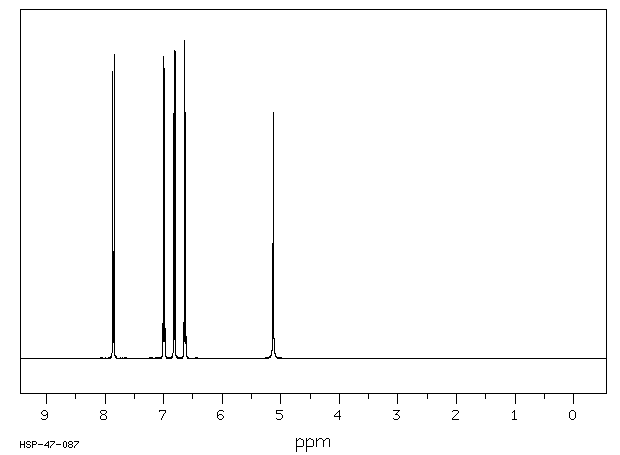
氢谱1HNMR
-
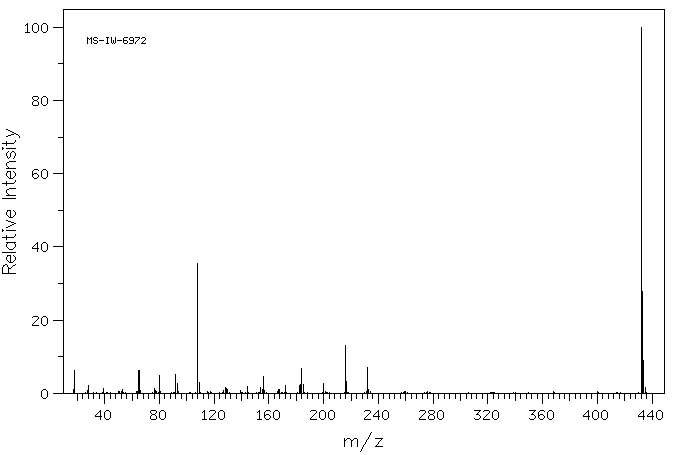
质谱MS
-
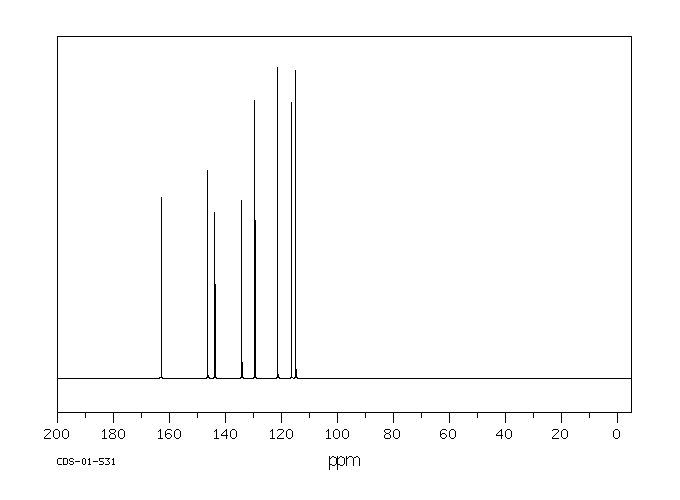
碳谱13CNMR
-
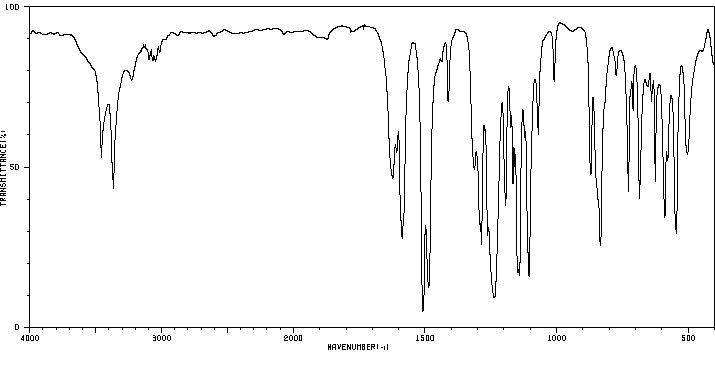
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫