2,2-二溴丙烷 | 594-16-1
中文名称
2,2-二溴丙烷
中文别名
二溴化丙酮
英文名称
2,2-dibromopropane
英文别名
——
CAS
594-16-1
化学式
C3H6Br2
mdl
——
分子量
201.889
InChiKey
ARITXYXYCOZKMU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-44.69°C (estimate)
-
沸点:114 °C (740 mmHg)
-
密度:1.78
-
闪点:113 °C
-
保留指数:754.2
-
稳定性/保质期:
避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xn,Xi
-
安全说明:S26,S36/37/39,S37/39
-
危险类别码:R36/37/38
-
海关编码:29033990
-
危险品运输编号:UN 2810
-
危险类别:6.1(b)
-
包装等级:III
-
储存条件:密封储存,应存放在阴凉、干燥的库房中。
SDS
| Name: | 2 2-Dibromopropane 98% Material Safety Data Sheet |
| Synonym: | Propane, 2,2-dibromo |
| CAS: | 594-16-1 |
Synonym:Propane, 2,2-dibromo
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 594-16-1 | 2,2-Dibromopropane | 98 | 209-828-9 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 594-16-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Clear liquid
Color: clear faint yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 114 deg C @ 740.00mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: 1.7820g/cm3
Molecular Formula: C3H6Br2
Molecular Weight: 201.89
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, light, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases, heat, direct light.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 594-16-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,2-Dibromopropane - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 594-16-1: No information available.
Canada
CAS# 594-16-1 is listed on Canada's NDSL List.
CAS# 594-16-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 594-16-1 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Reboul, Annales de Chimie (Cachan, France), 1878, vol. <5>14, p. 477;摘要:DOI:
-
作为产物:参考文献:名称:Kharasch; Kleiger; Mayo, Journal of Organic Chemistry, 1939, vol. 4, p. 431,434摘要:DOI:
-
作为试剂:描述:1-(3-hydroxyphenyl)-1-hexanol 、 2,2-二溴丙烷 、 potassium carbonate 在 2,2-二溴丙烷 、 sodium hydroxide 作用下, 以 丁酮 为溶剂, 反应 9.0h, 以There are obtained 25.8 g的产率得到1-Bromo-3-[3-(1-hydroxyhexyl)-phenoxy]-propane参考文献:名称:Hydroxamic acids and pharmaceutical compositions containing them, and摘要:本发明提供了一般式为:##STR1##其中Ar是苯基,可以选择用烷基,烯基,烷氧基,烷硫基,烷酰基,羟基,羟基烷基,羧基,烷氧羰基,氨基甲酰基,硝基,氨基或卤素进行取代,A是直链或支链,饱和或不饱和的C.sub.1-C.sub.8脂肪烃基,可以选择用羟基进行取代,n为0、1或2,B是直链或支链,饱和或不饱和的C.sub.1-C.sub.8脂肪烃基,R为5-(1H)-四唑基,--CO--NH-四唑基,##STR2##CN,SCN,OH,卤素,5-(1H)-四唑硫基,##STR3##或CO--O--NH--R.sub.1,其中R.sub.1是氢原子,烷基,可以选择用环烷基或烯基,环烷基,芳基烷基或芳基进行取代,其中芳香基可以选择用烷基,烯基,烷氧基,烷硫基,烷酰基,羟基,羟基烷基,羧基,烷氧羰基,氨基甲酰基,硝基,氨基或卤素进行取代;以及其药学上可接受的盐。本发明还提供了制备这些化合物的方法和含有它们的药物组合物,用于治疗过敏性疾病。公开号:US04906666A1
文献信息
-
Polarity Umpolung Strategy for the Radical Alkylation of Alkenes作者:Jige Liu、Shuo Wu、Jiajia Yu、Chenxi Lu、Zhen Wu、Xinxin Wu、Xiao‐Song Xue、Chen ZhuDOI:10.1002/anie.201915837日期:2020.5.18Free radical-mediated alkylation of general alkenes is a challenging and largely unmet goal. Herein, we disclose a conceptually novel "polarity umpolung" strategy for radical alkylation of alkenes using a portfolio of easily-accessed, dual-function alkylating reagents. This is achieved by substituting inherently nucleophilic alkyl radicals with electrophilic sulfone-decorated surrogates, thus inverting
-
Cyclopropanation of Dimethyl Fumarate and Maleate with<i>gem</i>-Dihalides Catalyzed by Co(0)- or Ni(0)-Complex and Zinc作者:Hiroyoshi Kanai、Yoshimasa Nishiguchi、Hideki MatsudaDOI:10.1246/bcsj.56.1592日期:1983.6The reaction of bis(acetonitrile)bis(diethyl fumarate)cobalt(0) with dibromomethane gave diethyl trans-1,2-cyclopropanedicarboxylate in a 67% yield based on the cobalt complex. Dimethyl cis-1,2-cyclopropanedicarboxylate was obtained in a 52% yield from the reaction of dimethyl maleate with dibromomethane in the presence of acetonitrilebis(dimethyl maleate)nickel(0) accompanied by the formation of the双(乙腈)双(富马酸二乙酯)钴(0)与二溴甲烷的反应得到反式-1,2-环丙烷二羧酸二乙酯,产率为67%,基于钴配合物。在乙腈双(马来酸二甲酯)镍 (0) 存在下,马来酸二甲酯与二溴甲烷反应,同时以 14% 的产率形成反式异构体,以 52% 的产率获得顺式-1,2-环丙烷二甲酸二甲酯. 添加锌以基于钴或镍的超过 100% 的产率得到环丙烷。3-甲基-和3,3-二甲基-1,2-环丙烷二甲酸酯是通过富马酸或马来酸二甲酯与1,1-二溴乙烷和2,2-二溴丙烷反应制备的。钴催化剂体系的产率随着墒二溴化物取代基的增加而降低,而在镍催化剂体系中的变化并不明显。少量异丙烯基琥珀酸二甲酯作为 2,2-二溴丙烷反应的副产物形成。简要讨论了一种机制,它...
-
9-Methylgermacrene-B is confirmed as the sex pheromone of the sandfly Lutzomyia longipalpis from Lapinha, Brazil, and the absolute stereochemistry defined as S作者:J. Gordon C. Hamilton、Helen C. Ibbotson、Antony M. Hooper、John A. PickettDOI:10.1039/a907910f日期:——The structure of the sex pheromone produced by the male sandfly Lutzomyia longipalpis, from the Lapinha Cave (Minas Gerais State) region of Brazil, previously proposed tentatively as the novel homosesquiterpene 9-methylgermacrene-B, is confirmed and the absolute stereochemistry defined as S by comparing physical and biological properties of the synthetic enantiomers with the natural product.
-
Chemoselective and stereoselective lithium carbenoid mediated cyclopropanation of acyclic allylic alcohols作者:M. J. Durán-Peña、M. E. Flores-Giubi、J. M. Botubol-Ares、L. M. Harwood、I. G. Collado、A. J. Macías-Sánchez、R. Hernández-GalánDOI:10.1039/c5ob02617b日期:——and the corresponding dihaloalkane has been evaluated. The reaction occurs in a chemo and stereoselective manner, which is consistent with a directing effect from the oxygen of the allylic moiety. Furthermore, a set of polyenes containing allylic hydroxyl or ether groups were chemoselectively and stereoselectively converted into the corresponding gem-dimethylcyclopropanes in one single step in moderate
-
Cobalt‐Catalyzed Reductive Dimethylcyclopropanation of 1,3‐Dienes作者:Jacob Werth、Christopher UyedaDOI:10.1002/anie.201807542日期:2018.10.15variant of the Simmons–Smith reaction that enables the efficient dimethylcyclopropanation of 1,3‐dienes using a Me2CCl2/Zn reagent mixture. The reactions proceed with high regioselectivity based on the substitution pattern of the 1,3‐diene. The products are vinylcyclopropanes, which serve as substrates for transition‐metal‐catalyzed ring‐opening reactions, including 1,3‐rearrangement and [5+2] cycloaddition
表征谱图
-
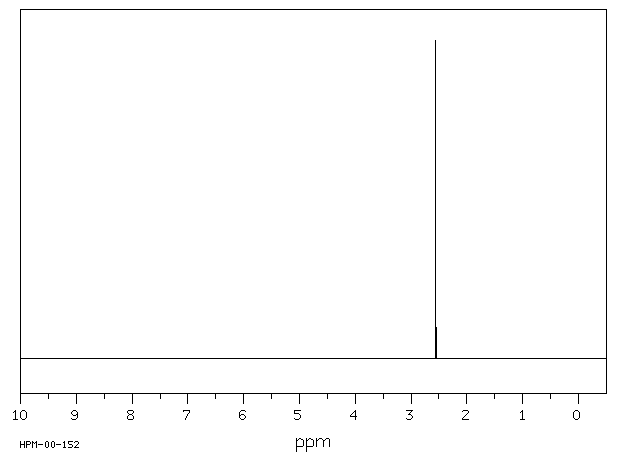
氢谱1HNMR
-
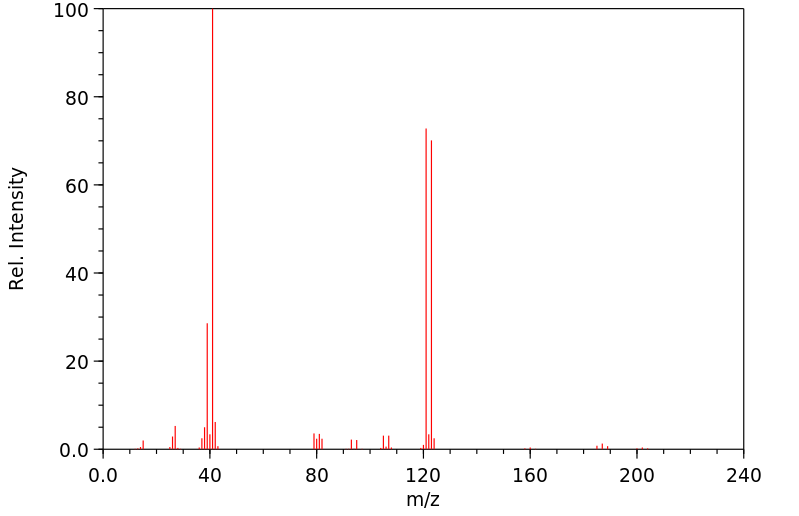
质谱MS
-
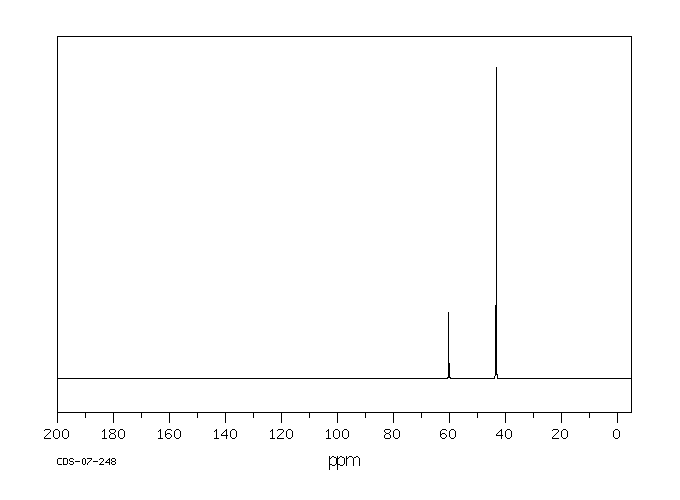
碳谱13CNMR
-
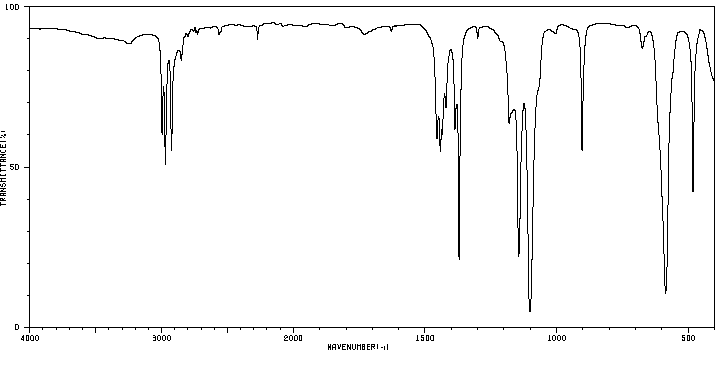
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(3-溴-1-丙炔-1-基)环丙烷
马杜拉霉素
顺-3,顺-6-1-溴壬二烯
顺,反,顺-1,2,3,4-四(2-溴乙基)环丁烷
金刚烷-2,2-d2
辛烷,1,5-二溴-
苯并噻唑,6-异硫氰酸根合5-甲基-(9CI)
苯(甲)醛,3-甲氧基-4-硝基-
硬脂基溴
硫杂二溴化
癸基溴
甲基环丙基溴化镁
环戊醇1-乙基-3-(苯甲基)-(9CI)
环戊烯-1,3-溴-(7CI,9CI)
环丙烷,1-溴-1-(3,3-二甲基-1-丁炔基)-2,2-二甲基-
环丁基溴
溴甲基环戊烷
溴甲基环己烷
溴甲基环丙烷
溴甲基环丁烷
溴甲基
溴环戊烷-D9
溴己烷-D3
溴己烷
溴化环辛基甲基
溴代环辛烷
溴代环戊烷
溴代环庚烷
溴代环丙烷
溴代异辛烷
溴代异丁烷
溴代叔丁烷-D9
溴代叔丁烷
溴代十四烷-D29
溴代十四烷
溴代十六烷-D33
溴代十六烷
溴代十五烷
溴代十二烷
溴代二十烷
溴乙醛
溴乙烷-D3
溴乙烷-D1
溴乙烷-2-13C
溴乙烷-13C2
溴乙烷-1-13C
溴乙烷-1,1-d2
溴乙烷-1,1,2,2-d4
溴乙烷
溴丙烷-D4