己醛而甲基乙缩醛 | 1599-47-9
中文名称
己醛而甲基乙缩醛
中文别名
1,1-二甲氧基己烷;己醛二甲基乙缩醛;己醛二甲基乙缩醛,98%
英文名称
1,1-dimethoxyhexane
英文别名
hexanal dimethyl acetal
CAS
1599-47-9
化学式
C8H18O2
mdl
MFCD00036508
分子量
146.23
InChiKey
ZQUJUCJLDXTKKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:163-164 °C(lit.)
-
密度:0.847 g/mL at 25 °C(lit.)
-
闪点:130 °F
-
LogP:2.041 (est)
-
保留指数:964
-
稳定性/保质期:
常温常压下稳定,避免氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:10
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R10
-
WGK Germany:3
-
海关编码:2911000000
-
包装等级:III
-
危险品运输编号:UN 1993 3
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:简单的一锅法将醛转化为甲酯摘要:Several methyl esters were obtained by an efficient and simple one-pot procedure from the corresponding aldehydes in high yields. This procedure involves dimethyl acetal formation from aldehydes and subsequent oxidation. (C) 1998 Elsevier Science Ltd. All rights reserved.DOI:10.1016/s0040-4039(97)10845-0
-
作为产物:参考文献:名称:Palladium-catalyzed triethylammonium formate reductions. 4. Reduction of acetylenes to cis-monoenes and hydrogenolysis of tertiary allylic amines摘要:DOI:10.1021/jo01312a021
文献信息
-
An Efficient Method for One-Pot Reductive Cleavage of Acetals to Primary Alcohols Using a Bimetal Redox Couple CoCl2.6H2O-Zn作者:Kuladip Sarma、Amrit GoswamiDOI:10.2174/157017809789869456日期:2009.10.1Acyclic or cyclic O,O-acetals and O,S-acetals underwent reductive cleavage to give primary alcohols efficiently on treatment with Zn-CoCl2.6H2O-bimetal redox system in dry tetrahydrofuran at ambient temperature to give good to excellent yields.
-
Efficient synthesis of acetals catalysed by rare earth chlorides
-
The Catalytic Reduction of Aldehydes and Ketones with 2-Propanol over Hydrous Zirconium Oxide作者:Makoto Shibagaki、Kyoko Takahashi、Hajime MatsushitaDOI:10.1246/bcsj.61.3283日期:1988.9Reduction of aldehydes with 2-propanol proceeded efficiently by catalysis with hydrous zirconium oxide to give the corresponding alcohols. Most ketones also were reduced efficiently, but conjugated or sterically hindered ketones resisted the reduction. The reduction was carried out with primary, secondary, or tertiary alcohols, and only secondary alcohols served as hydrogen donors. Kinetic experiments
-
A New Method for the Preparation of δ-Alkoxy-α,β-unsaturated Aldehydes and Polyenals作者:Akihiko Ishida、Teruaki MukaiyamaDOI:10.1246/bcsj.50.1161日期:1977.5TiCl4 and also in the coexistence of TiCl4 and Ti(OiPr)4 to give δ-alkoxy-α,β-unsaturated aldehydes (3–5) in good yields. In the presence of 1,8-diazabicyclo[5.4.0] undec-7-ene or 1,5-diazabicyclo[4.3.0]non-5-ene and molecular sieves 3A or 4A, the elimination reaction of δ-alkoxyl group of α,β-unsaturated aldehydes (3–5) proceeds smoothly to afford the corresponding polyenals in good yields.二烯氧基硅烷 (2) 在 TiCl4 存在下以及 TiCl4 和 Ti(OiPr)4 共存下与缩醛反应,以良好的产率得到 δ-烷氧基-α,β-不饱和醛 (3-5)。在1,8-二氮杂双环[5.4.0]十一碳-7-烯或1,5-二氮杂双环[4.3.0]非5-烯和分子筛3A或4A存在下,δ-烷氧基的消除反应α,β-不饱和醛 (3-5) 的合成顺利进行,以良好的收率提供相应的多烯醛。
-
Novel Preparation of α,β-Unsaturated Aldehydes. Benzeneselenolate Promotes Elimination of HBr from α-Bromoacetals作者:Andrei Vasil'ev、Lars EngmanDOI:10.1021/jo9917644日期:2000.4.1elimination/hydrolysis of these mixtures afforded alpha,beta-unsaturated aldehydes in 50-80% overall yields. In the case of tertiary alpha-bromoacetals, treatment with benzeneselenolate afforded only dehydrobromination products as mixtures of isomers. The presence of at least a catalytic amount of the organoselenium reagent was found to be crucial for olefin formation. A SET-mechanism, involving benzeneselenolate-induced研究了乙酰化,α-溴化,亲核性苯硒基化,氧化消除/水解作为醛类α,β-脱氢的新方法。用溴在二氯甲烷中的乙缩醛处理得到相应的α-溴缩醛,产率为80-90%。然后,通过用由二苯基二硒化物,肼和碳酸钾在二甲基亚砜中原位生成的苯硒酸酯处理,可以方便地实现亲核性苯基硒烯基化。无支链的α-溴缩醛可干净地提供取代产物,而β和γ支链的则通过形式上的溴化氢损失而产生大量的α,β-不饱和缩醛。这些混合物的氧化消除/水解以50-80%的总产率提供α,β-不饱和醛。在叔α-溴缩醛的情况下,用苯硒烯酸酯处理仅得到作为异构体混合物的脱氢溴化产物。发现至少催化量的有机硒试剂的存在对于烯烃形成至关重要。提出了一种SET机制,包括苯硒酸酯诱导的电子转移到卤化物,溴离子的损失以及氢原子或质子/电子,用于苯甲酸酯促进的消除反应。旨在在分子内环化或开环反应中捕获以碳为中心的自由基的实验未能提供任何自由基中间体的证据。发现至少催化
表征谱图
-
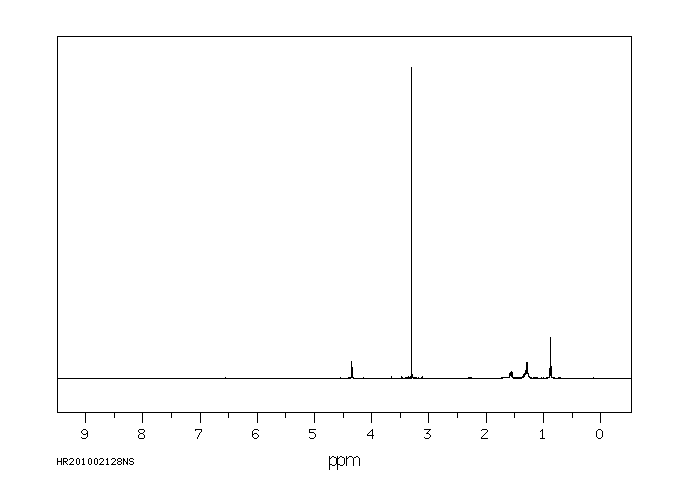
氢谱1HNMR
-
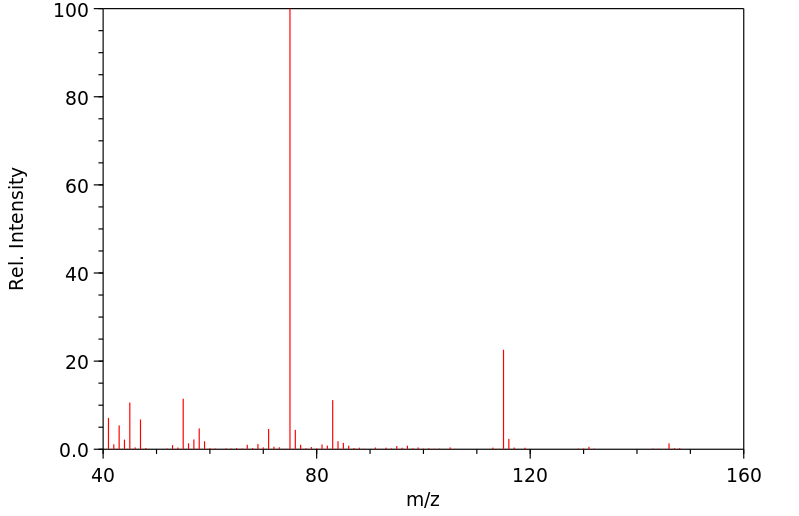
质谱MS
-
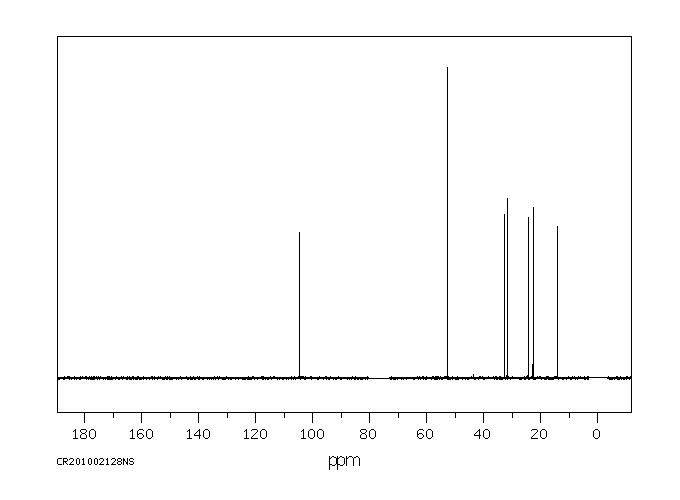
碳谱13CNMR
-
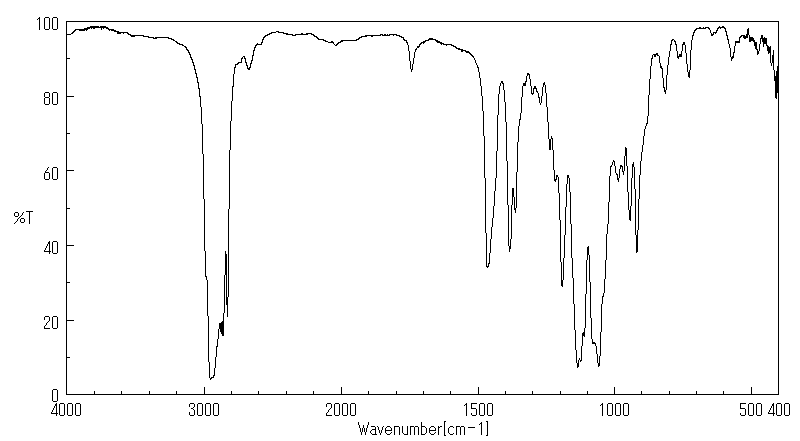
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷