4-溴邻苯二甲酰亚胺 | 6941-75-9
中文名称
4-溴邻苯二甲酰亚胺
中文别名
5-溴异吲哚-1,3-二酮;4-溴酞酰亚胺
英文名称
5-Bromoisoindoline-1,3-dione
英文别名
4-bromophthalimide;5-bromoisoindole-1,3-dione;5-bromophthalimide;5-bromopthalamide;5-bromo-1,3-dihydro-1,3-dioxo-2H-isoindole
CAS
6941-75-9
化学式
C8H4BrNO2
mdl
MFCD00466049
分子量
226.029
InChiKey
GNYICZVGHULCHE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:235 °C
-
密度:1.826±0.06 g/cm3(Predicted)
-
溶解度:几乎全溶于N,N-DMF溶液中。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:46.2
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S26,S36/37/39
-
海关编码:2925190090
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放于室温环境中,并请密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
4-Bromophthalimide
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
4-Bromophthalimide
Ingredient name:
CAS number: 6941-75-9
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H4BrNO2
Molecular weight: 226.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
4-Bromophthalimide
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
4-Bromophthalimide
Ingredient name:
CAS number: 6941-75-9
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C8H4BrNO2
Molecular weight: 226.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质:黄色至浅黄色粉末
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 邻苯二甲酸亚胺 phthalimide 85-41-6 C8H5NO2 147.133 4-氨基邻苯二甲酰亚胺 4-aminophthalimide 3676-85-5 C8H6N2O2 162.148 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-溴-N-甲基酞亚胺 5-bromo-2-methylisoindoline-1,3-dione 90224-73-0 C9H6BrNO2 240.056 —— 5-bromo-2-ethylisoindoline-1,3-dione —— C10H8BrNO2 254.083 4-溴邻苯二甲酰胺 4-Bromophthalamid 490038-15-8 C8H7BrN2O2 243.06 —— 5-bromo-2-isopropylisoindoline-1,3-dione 351996-68-4 C11H10BrNO2 268.11 —— 5-bromo-2-(2-oxopropyl)isoindolyl-1,3-dione —— C11H8BrNO3 282.093 —— 5-bromo-2-pyridin-3-ylmethyl-isoindole-1,3-dione 348125-24-6 C14H9BrN2O2 317.142 —— 4-(5-bromo-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)butanoic acid 299964-12-8 C12H10BrNO4 312.12 5-溴-3-羟基异吲哚啉-1-酮 5-bromo-3-hydroxyisoindoline-1-one 573675-39-5 C8H6BrNO2 228.045 —— (5-bromo-1,3-dioxo-1,3-dihydro-isoindol-2-yl)-acetic acid methyl ester 348130-03-0 C11H8BrNO4 298.093 —— (5-bromo-1,3-dioxo-1,3-dihydro-isoindol-2-yl)-acetic acid ethyl ester 351895-37-9 C12H10BrNO4 312.12 5-溴异二氢吲哚 5-bromoisoindoline 127168-84-7 C8H8BrN 198.062 5-溴-6-硝基异吲哚啉-1,3-二酮 5-bromo-6-nitro-isoindole-1,3-dione 64823-14-9 C8H3BrN2O4 271.027 5-氰基邻苯二甲酰亚胺 1,3-dioxoisoindoline-5-carbonitrile 34613-09-7 C9H4N2O2 172.143 —— 5-bromo-2-(2-(7-methoxynaphthalen-1-yl)ethyl)isoindoline-1,3-dione 1595280-08-2 C21H16BrNO3 410.267 —— 5-(phenylethynyl)o-benzenedicarboximide 1092550-39-4 C16H9NO2 247.253 5-苯基-1H-异吲哚-1,3(2H)-二酮 3,4-biphenyldicarboxyimide 2021-26-3 C14H9NO2 223.231 —— 4-(N,N-dimethylamino)phthalimide 90915-39-2 C10H10N2O2 190.202 —— 4-(phenylthio)phthalimide 343223-65-4 C14H9NO2S 255.297 —— 5-((4-Fluorophenyl)ethynyl)isoindoline-1,3-dione 1283696-04-7 C16H8FNO2 265.243 —— 5-bromo-2-mesitylisoindoline-1,3-dione —— C17H14BrNO2 344.208 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:四(二乙基膦基)-,四(乙基苯基膦基)-和四(二苯基膦基氧化物)-取代酞菁摘要:四(膦酸二乙酯),四(苯基次膦酸乙酯)和四(二苯基膦氧化物)取代的酞菁DOI:10.1002/hlca.200490081
-
作为产物:参考文献:名称:四(二乙基膦基)-,四(乙基苯基膦基)-和四(二苯基膦基氧化物)-取代酞菁摘要:四(膦酸二乙酯),四(苯基次膦酸乙酯)和四(二苯基膦氧化物)取代的酞菁DOI:10.1002/hlca.200490081
-
作为试剂:描述:1,3-dioxoisoindolin-2-yl (tert-butoxycarbonyl)valinate 在 4-溴邻苯二甲酰亚胺 、 4-(三氟甲基)苯硫酚 、 caesium carbonate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 6.0h, 以76%的产率得到tert-butyl (1-(5-bromo-1,3-dioxoisoindolin-2-yl)-2-methylpropyl)carbamate参考文献:名称:N-(乙酰氧基)邻苯二甲酰亚胺的硫酚催化可见光光氧化还原脱羧偶联摘要:我们已经开发了在不添加光催化剂的情况下N-(乙酰氧基)邻苯二甲酰亚胺的可见光光氧化还原脱羧偶合,其中简单的和可商购的苯酚被用作有效的有机催化剂,而4-(三氟甲基)苯硫酚显示出最佳的催化活性。选择了三个代表性的脱羧实例,包括一个氨基化和两个C–C键偶联,以确认可见光氧化还原反应的功效,结果表明它们在室温下表现良好。有趣的发现应该为有机分子的可见光光氧化还原转化提供一种新颖且环保的策略。DOI:10.1021/acs.orglett.6b03300
文献信息
-
Certain pyrazoline derivatives with kinase inhibitory activity申请人:Adams Ruth S.公开号:US20080171754A1公开(公告)日:2008-07-17The present invention provides certain pyrazoline compounds useful as inhibitors of protein kinases. The invention also provides pharmaceutical compositions and methods of using the compositions in the treatment of various diseases.
-
CHEMICAL COMPOUNDS申请人:Deng Jianghe公开号:US20090143372A1公开(公告)日:2009-06-04The invention is directed to novel indole carboxamide derivatives. Specifically, the invention is directed to compounds according to formula I: where R1, R2, R3, U and V are defined below and to pharmaceutically acceptable salts thereof. The compounds of the invention are inhibitors of IKK2 and can be useful in the treatment of disorders associated with inappropriate IKK2 (also known as IKKβ) activity, such as rheumatoid arthritis, asthma, and COPD (chronic obstructive pulmonary disease). Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting IKK2 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
-
Multicyclic bis-amide MMP inhibitors申请人:Powers Timothy公开号:US20060173183A1公开(公告)日:2006-08-03The present invention relates generally to bis-amide group containing pharmaceutical agents, and in particular, to multicyclic bis-amide MMP-13 inhibitor compounds. More particularly, the present invention provides a new class of MMP-13 inhibiting compounds, containing a pyrimidinyl bis-amide group in combination with a heterocyclic moiety, that exhibit an increased potency and solubility in relation to currently known bis-amide group containing MMP-13 inhibitors.
-
[EN] MODULATORS OF THE HISTAMINE H3 RECEPTOR USEFUL FOR THE TREATMENT OF DISORDERS RELATED THERETO<br/>[FR] MODULATEURS DU RÉCEPTEUR D'HISTAMINE H3 UTILES POUR LE TRAITEMENT D'AFFECTIONS QUI LUI SONT ASSOCIÉES申请人:ARENA PHARM INC公开号:WO2009105206A1公开(公告)日:2009-08-27Amide derivatives of Formula (Ia) and pharmaceutical compositions thereof that modulate the activity of the histamine H3 receptor. Compounds of the present invention and pharmaceutical compositions thereof are directed to methods useful in the treatment of histamine H3-associated disorders, such as cognitive disorders, epilepsy, brain trauma, depression, obesity, disorders of sleep and wakefulness such as excessive daytime sleepiness, narcolepsy, shift-work sleep disorder, drowsiness as a side effect from a medication, maintenance of vigilance to aid in the completion of tasks and the like, cataplexy, hypersomnia, somnolence syndrome, jet lag, sleep apnea and the like, attention deficit hyperactivity disorder (ADHD), schizophrenia, allergies, allergic responses in the upper airway, allergic rhinitis, nasal congestion, dementia, Alzheimer's disease, pain and the like.
-
The Art of Filling Protein Pockets Efficiently with Octahedral Metal Complexes作者:Sebastian Blanck、Jasna Maksimoska、Julia Baumeister、Klaus Harms、Ronen Marmorstein、Eric MeggersDOI:10.1002/anie.201108865日期:2012.5.21Better fit, less effort: An easy‐to‐synthesize ruthenium phthalimide complex (tan‐colored carbon atoms in the picture) was designed to bind within the active site of the p21‐activated kinase 1 in a novel fashion that differs from that of the previously established staurosporine‐inspired metallopyridocarbazoles (gray‐colored carbon atoms).
表征谱图
-
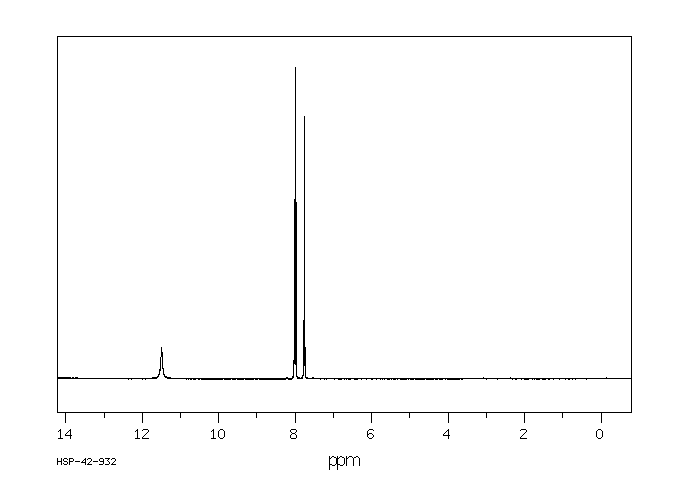
氢谱1HNMR
-
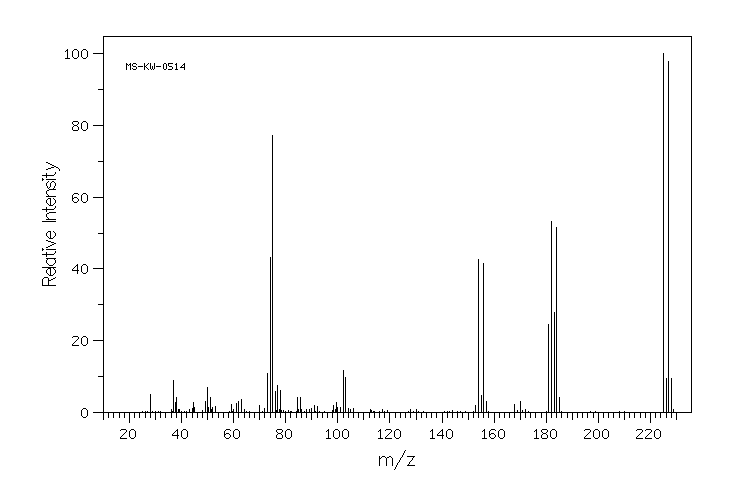
质谱MS
-
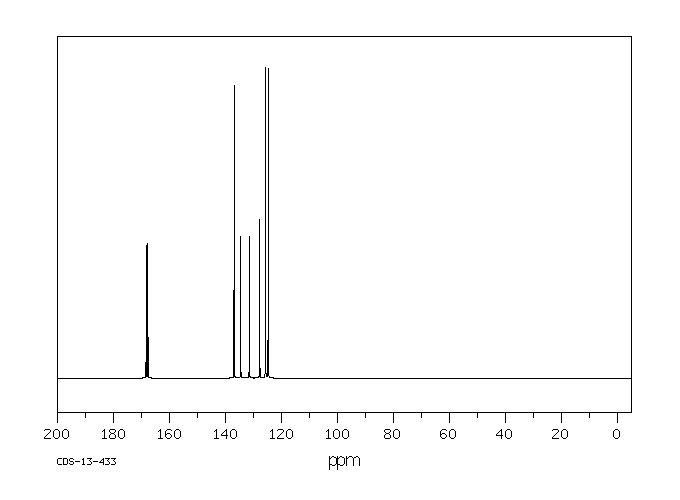
碳谱13CNMR
-
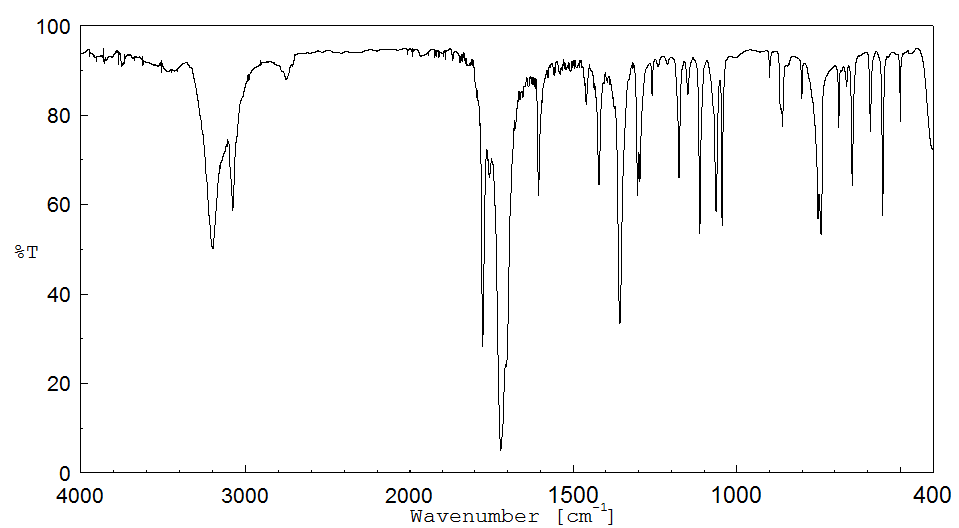
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1Z,3Z)-1,3-双[[((4S)-4,5-二氢-4-苯基-2-恶唑基]亚甲基]-2,3-二氢-5,6-二甲基-1H-异吲哚
鲁拉西酮杂质33
鲁拉西酮杂质07
马吲哚
颜料黄110
顺式-六氢异吲哚盐酸盐
顺式-2-[(1,3-二氢-1,3-二氧代-2H-异吲哚-2-基)甲基]-N-乙基-1-苯基环丙烷甲酰胺
顺式-2,3,3a,4,7,7a-六氢-1H-异吲哚
顺-N-(4-氯丁烯基)邻苯二甲酰亚胺
降莰烷-2,3-二甲酰亚胺
降冰片烯-2,3-二羧基亚胺基对硝基苄基碳酸酯
降冰片烯-2,3-二羧基亚胺基叔丁基碳酸酯
阿胍诺定
阿普斯特降解杂质
阿普斯特杂质FA
阿普斯特杂质68
阿普斯特杂质29
阿普斯特杂质27
阿普斯特杂质26
阿普斯特杂质19
阿普斯特杂质08
阿普斯特杂质03
阿普斯特杂质
阿普斯特二聚体杂质
阿普斯特
防焦剂MTP
铝酞菁
铁(II)1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-十六氟-29H,31H-酞菁
铁(II)2,9,16,23-四氨基酞菁
钠S-(2-{[2-(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙基]氨基}乙基)氢硫代磷酸酯
酞酰亚胺-15N钾盐
酞菁锡
酞菁二氯化硅
酞菁 单氯化镓(III) 盐
酞美普林
邻苯二甲酸亚胺
邻苯二甲酰基氨氯地平
邻苯二甲酰亚胺,N-((吗啉)甲基)
邻苯二甲酰亚胺阴离子
邻苯二甲酰亚胺钾盐
邻苯二甲酰亚胺钠盐
邻苯二甲酰亚胺观盐
邻苯二亚胺甲基磷酸二乙酯
那伏莫德
过氧化氢,2,5-二氢-5-苯基-3H-咪唑并[2,1-a]异吲哚-5-基
达格吡酮
诺非卡尼
螺[环丙烷-1,1'-异二氢吲哚]-3'-酮
螺[异吲哚啉-1,4'-哌啶]-3-酮盐酸盐
葡聚糖凝胶G-25