2-乙酰-5-甲基噻吩 | 13679-74-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:24-28 °C(lit.)
-
沸点:65-67 °C1 mm Hg(lit.)
-
密度:1.106 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
LogP:2.04
-
物理描述:Solid
-
溶解度:Very slightly soluble in water
-
折光率:1.557-1.567
-
保留指数:1123;1130;1185;1170;1174;1204.9
-
稳定性/保质期:
常温常压下稳定,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.285
-
拓扑面积:45.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:STENCH
-
危险品标志:Xn,Xi
-
安全说明:S22,S36/37
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2934999090
-
危险品运输编号:UN 3335
-
RTECS号:OB4972000
-
危险类别:STENCH
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:常温下应密闭保存,在阴凉、通风和干燥处存放。
SDS
模块 1. 化学品
1.1 产品标识符
: 2-乙酰基-5-甲基噻吩
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物
恶臭
模块 3. 成分/组成信息
3.1 物 质
: C7H8OS
分子式
: 140.2 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 24 - 28 °C - lit.
f) 沸点、初沸点和沸程
65 - 67 °C 在 1 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.106 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 腹膜内的 - 小鼠 - 230 mg/kg
备注: 行为的:抽搐或对癫痫阈值的影响。 肺,胸,或者呼吸系统:其他变化
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: OB4972000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 3335
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: Aviation regulated solid, n.o.s. (1-(5-Methyl-2-thienyl)ethan-1-one)
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: 9
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
2-乙酰基-5-甲基噻吩是咖啡香气的成分之一。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-乙酰基-5-氯噻酚 2-acetyl-5-chlorothiophene 6310-09-4 C6H5ClOS 160.624 2-乙酰基-5-溴噻吩 2-Acetyl-5-bromothiophene 5370-25-2 C6H5BrOS 205.075 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-bromo-1-(5-methylthiophen-2-yl)-ethan-1-one 10531-42-7 C7H7BrOS 219.102 2-氯-1-(5-甲基-噻吩-2-基)-乙酮 2-chloro-1-(5-methylthiophen-2-yl)ethan-1-one 31772-42-6 C7H7ClOS 174.651 —— 1-(5-methylthien-2-yl)-2-hydroxyethanone 132706-13-9 C7H8O2S 156.205 5-乙酰基噻吩-2-甲酸 5-acetylthiophene-2-carboxylic acid 4066-41-5 C7H6O3S 170.189 1-(4-氯-5-甲基噻吩-2-基)乙酮 1-(4-Chlor-5-methyl-2-thienyl)-ethanon 123418-42-8 C7H7ClOS 174.651 —— methyl 5-Azidomethyl-thien-2-yl Ketone 69213-77-0 C7H7N3OS 181.218 1-(4-溴-5-甲基噻吩-2-基)乙酮 1-(4-bromo-5-methylthiophen-2-yl)ethan-1-one 36901-17-4 C7H7BrOS 219.102 —— 2-(3'-dimethylamino-1'-oxo-2'-propen-1'-yl)-5-methylthiopene 90815-53-5 C10H13NOS 195.285 —— 3-dimethylamino-1-(5-methyl-2-thienyl)-propanone 105398-49-0 C10H15NOS 197.301 2-乙基-5-甲基噻吩 2-ethyl-5-methylthiophene 40323-88-4 C7H10S 126.222 —— (5-methyl-thiophen-2-yl)-oxo-acetic acid 50845-89-1 C7H6O3S 170.189 —— (2E,4E)-5-(dimethylamino)-1-(5-methylthiophen-2-yl)penta-2,4-dien-1-one 75143-39-4 C12H15NOS 221.323 —— 2-trans-cinnamoyl-5-methyl-thiophene 26903-26-4 C14H12OS 228.315 —— 1-(5-methylthiophen-2-yl)-3-(thiophen-2-yl)propenone 943248-14-4 C12H10OS2 234.343 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Herbizide Thienylharnstoffe, II摘要:DOI:10.1007/bf00809650
-
作为产物:描述:在 sodium hydroxide 作用下, 生成 2-乙酰-5-甲基噻吩参考文献:名称:一系列4-和5-取代的2-([[ 3 H]乙酰基)噻吩的破坏动力学摘要:制备了一系列的4-和5-取代的2-([[ 3 H]乙酰基)噻吩,并在25.0°C下研究了氢氧化物催化的降解动力学。二阶降解速率常数与在相应的间位和对位取代的苯乙酮中观察到的平行,但随着反应常数ρ较高(1.61),反应范围比对苯乙酮所观察到的要宽得多。DOI:10.1039/p29850000557
文献信息
-
[EN] CYCLIC SULFAMIDE COMPOUNDS AND METHODS OF USING SAME<br/>[FR] COMPOSÉS DE SULFAMIDE CYCLIQUE ET LEURS PROCÉDÉS D'UTILISATION申请人:ASSEMBLY BIOSCIENCES INC公开号:WO2018160878A1公开(公告)日:2018-09-07The present disclosure provides, in part, cyclic sulfamide compounds, and pharmaceutical compositions thereof, useful as modulators of Hepatitis B (HBV) core protein, and methods of treating Hepatitis B (HBV) infection.本公开提供了部分环磺胺化合物及其药物组合物,可用作乙型肝炎(HBV)核心蛋白的调节剂,并用于治疗乙型肝炎(HBV)感染的方法。
-
Synthesis, characterization and anticancer activity of new 2-acetyl-5-methyl thiophene and cinnamaldehyde thiosemicarbazones and their palladium(II) complexes作者:Eunice A. Nyawade、Nicole R.S. Sibuyi、Mervin Meyer、Roger Lalancette、Martin O. OnaniDOI:10.1016/j.ica.2020.120036日期:2021.1Abstract New thiosemicarbazone (TSC) ligands, AMT-C N-TSCH (L1), AMT-C N-TSC(CH3) (L2), CIN-C N-TSCH (L3) and CIN-C N-TSC(CH3) (L4) (AMT = 2-acetyl-5-methylthiophene, TSCH = thiosemicarbazide, TSC(CH3) = 4-methyl-3-thiosemicarbazide, CIN = cinnamaldehyde) were synthesized by condensation reaction. The reaction of [PdCODCl2] with the ligands L1, L2 and L4in the ratio 1:1 yielded complexes [Pd(L1)Cl]摘要新的硫半脲(TSC)配体,AMT-C N-TSCH(L1),AMT-C N-TSC(CH3)(L2),CIN-C N-TSCH(L3)和CIN-C N-TSC( )通过缩合反应合成了(L4)(AMT = 2-乙酰基-5-甲基噻吩,TSCH =硫代氨基脲,TSC( )= 4-甲基-3-硫代氨基脲,CIN =肉桂醛)。[PdCODCL2]与配体L1,L2和L4的比例为1:1的反应生成了[Pd(L1)Cl](C1),[Pd(L2)CL2](C2)和[Pd(L4)2CL2分别为(C4)(COD = 1,5-环辛二烯)。[Pd(COD)CL2]与CIN-C N-TSCH(L3)在金属与配体比率为1:2的反应中生成复合物[Pd(L3)2](C3)。通过UV-Vis,FT-IR,NMR,元素分析和电导率测量来表征配体和配合物。配体L1以三齿方式配位至金属,而L2,L3和L4以二齿方式配位。配
-
Development of a Strategy for Linear-Selective Cu-Catalyzed Reductive Coupling of Ketones and Allenes for the Synthesis of Chiral γ-Hydroxyaldehyde Equivalents作者:Raphael K. Klake、Samantha L. Gargaro、Skyler L. Gentry、Sharon O. Elele、Joshua D. SieberDOI:10.1021/acs.orglett.9b02973日期:2019.10.4N-substituted allyl equivalents generated from a chiral allenamide. By choice of the appropriate ligand for the Cu-catalyst, high linear selectivity can be obtained with good diastereocontrol. This methodology allows access to chiral γ-hydroxyaldehyde equivalents that were applied in the synthesis of chiral γ-lactones and 2,5-disubstitued tetrahydrofurans.
-
Synthesis and Characterization of AlCl<sub>3</sub> Impregnated Molybdenum Oxide as Heterogeneous Nano-Catalyst for the Friedel-Crafts Acylation Reaction in Ambient Condition作者:Arvind H. Jadhav、Amutha Chinnappan、Vishwanath Hiremath、Jeong Gil SeoDOI:10.1166/jnn.2015.11253日期:2015.10.1
Aluminum trichloride (AlCl3) impregnated molybdenum oxide heterogeneous nano-catalyst was prepared by using simple impregnation method. The prepared heterogeneous catalyst was characterized by powder X-ray diffraction, FT-IR spectroscopy, solid-state NMR spectroscopy, SEM imaging, and EDX mapping. The catalytic activity of this protocol was evaluated as heterogeneous catalyst for the Friedel-Crafts acylation reaction at room temperature. The impregnated MoO4(AlCl2)2 catalyst showed tremendous catalytic activity in Friedel-Crafts acylation reaction under solvent-free and mild reaction condition. As a result, 84.0% yield of acyl product with 100% consumption of reactants in 18 h reaction time at room temperature was achieved. The effects of different solvents system with MoO4(AlCl2)2 catalyst in acylation reaction was also investigated. By using optimized reaction condition various acylated derivatives were prepared. In addition, the catalyst was separated by simple filtration process after the reaction and reused several times. Therefore, heterogeneous MoO4(AlCl2)2 catalyst was found environmentally benign catalyst, very convenient, high yielding, and clean method for the Friedel-Crafts acylation reaction under solvent-free and ambient reaction condition.
氯化铝(AlCl3)浸渍的钼氧化物非均相纳米催化剂是通过简单的浸渍方法制备的。所制备的非均相催化剂通过粉末X射线衍射、傅里叶变换红外光谱、固体核磁共振光谱、扫描电镜成像和能谱分析进行了表征。该方法的催化活性被评估为在室温下作为弗里德尔-克拉夫茨酰化反应的非均相催化剂。浸渍的MoO4(AlCl2)2催化剂在无溶剂和温和反应条件下表现出巨大的催化活性。结果表明,在室温下,18小时反应时间内,酰基产物的产率为84.0%,反应物的消耗率为100%。还研究了在酰化反应中使用 ( )2催化剂的不同溶剂体系的影响。通过使用优化的反应条件,制备了各种酰化衍生物。此外,反应后通过简单的过滤过程分离催化剂,并多次重复使用。因此,非均相 ( )2催化剂被发现是一种环境友好的催化剂,非常方便,产率高,是一种无溶剂和环境条件下进行弗里德尔-克拉夫茨酰化反应的清洁方法。 -
Cross-Coupling Reaction with Lithium Methyltriolborate作者:Yasunori Yamamoto、Kazuya Ikizakura、Hajime Ito、Norio MiyauraDOI:10.3390/molecules18010430日期:——We newly developed lithium methyltriolborate as an air-stable white solid that is convenient to handle. The good performance of this triolborate for metal-catalyzed bond-forming reactions was demonstrated in palladium-catalyzed cross-coupling reactions with haloarenes. Cross-coupling reaction of [MeB(OCH2)3CCH3]Li with aryl halides occurred in the presence of Pd(OAc)2/RuPhos complex in refluxing MeOH/H2O and the absence of bases.
表征谱图
-
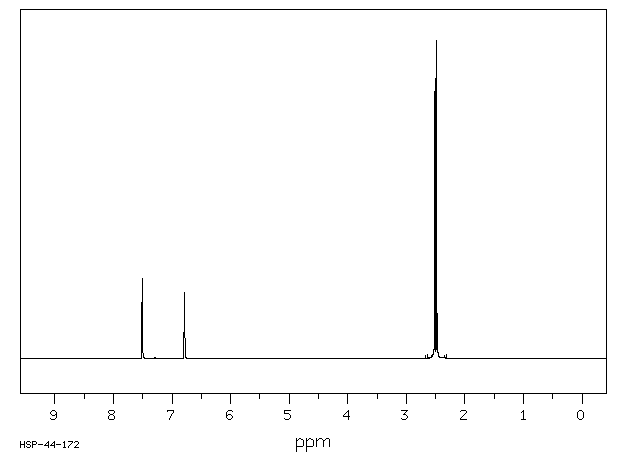
氢谱1HNMR
-
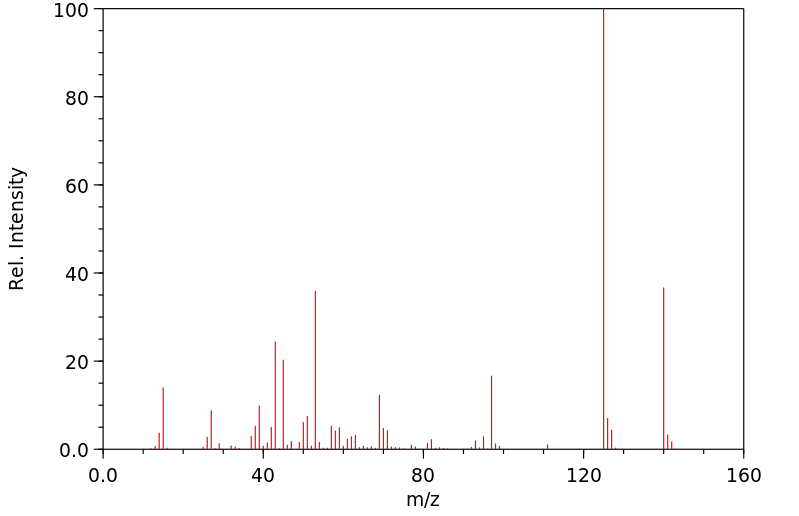
质谱MS
-
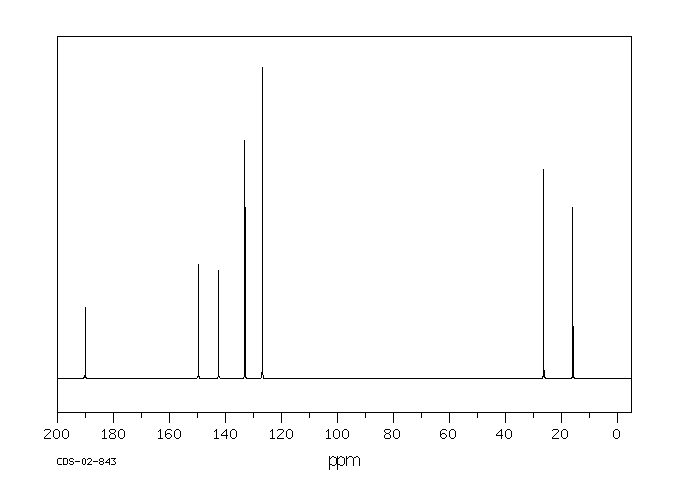
碳谱13CNMR
-
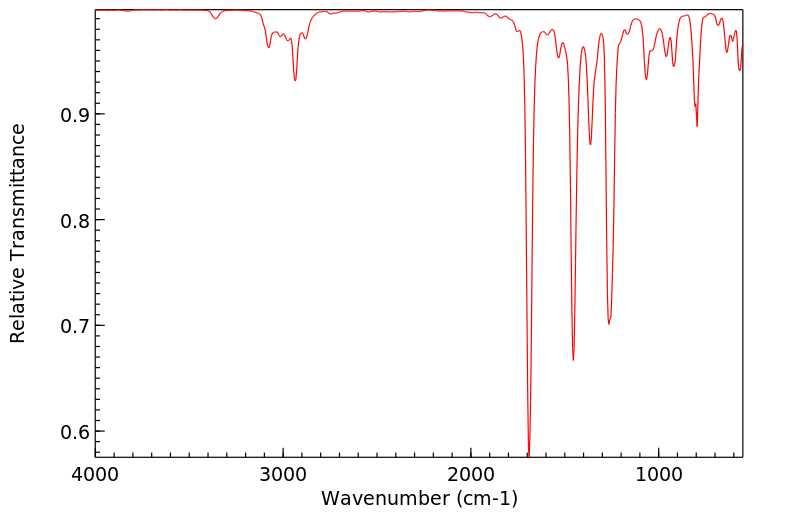
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息