2-丁基苯胺 | 2696-85-7
中文名称
2-丁基苯胺
中文别名
2-N-丁基苯胺;2-正丁基苯胺
英文名称
2-butyl-benzenamine
英文别名
2-n-butylaniline;2-butylaniline
CAS
2696-85-7
化学式
C10H15N
mdl
MFCD00130033
分子量
149.236
InChiKey
HDVUPIFFKAHPJY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:126 °C / 15mmHg
-
密度:0,95 g/cm3
-
保留指数:1390
-
稳定性/保质期:
遵循规定使用和储存,则不会发生分解。
计算性质
-
辛醇/水分配系数(LogP):2.8
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:26
-
氢给体数:1
-
氢受体数:1
安全信息
-
安全说明:S23,S36/37/39
-
危险类别码:R20/21/22
-
海关编码:2921420090
-
WGK Germany:3
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放于阴凉干燥处
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2-n-Butylaniline
Portland OR
Synonym Not available.
Chemical Formula NH2C6H4(CH2)3CH3
CAS Number 2696-85-7
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2-n-Butylaniline 2696-85-7 Min. 95.0 Not available. Not available.
(GC)
Section III. Hazards Identification
Acute Health Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least 15
minutes. Get medical attention.
Skin Contact
In case of contact, immediately flush skin with plenty of water. Remove contaminated clothing and shoes. Wash clothing
before reuse. Thoroughly clean shoes before reuse. Get medical attention.
If the victim is not breathing, perform mouth-to-mouth resuscitation. Loosen tight clothing such as a collar, tie, belt or
Inhalation
waistband. If breathing is difficult, oxygen can be administered. Seek medical attention if respiration problems do not
improve.
Ingestion INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not breathing, perform mouth-to-mouth
resuscitation. Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the
toxic material was ingested; the absence of such signs, however, is not conclusive. SEEK IMMEDIATE MEDICAL
ATTENTION in case of ingestion of a radioactive material.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flash Points Flammable Limits Not available.
108°C (226.4°F).
Combustion Products
These products are toxic carbon oxides (CO, CO 2), nitrogen oxides (NO, NO2).
Fire Hazards
Not available.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media
SMALL FIRE: Use DRY chemical powder.
LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
and Instructions
Continued on Next Page
2-n-Butylaniline
Section VI. Accidental Release Measures
Spill Cleanup Irritating material.
Instructions In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and excercise caution. Use a
shovel to put material into a convenient waste disposal container. Finish cleaning the spill by rinsing any contaminated
surfaces with copious amounts of water. Consult federal, state and/or local authorities for assistance on disposal.
Section VII. Handling and Storage
Handling and Storage IRRITANT. Keep away from heat. Mechanical exhaust required. When not in use, tightly seal the container and store in
a dry, cool place. Avoid excessive heat and light. Do not breathe gas/fumes/ vapor/spray.
Information
Always store away from incompatible compounds such as oxidizing agents, acids.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor respirator. Boots. Gloves. Be sure to use a MSHA/NIOSH approved respirator or
equivalent. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. Not available.
0.953
Specific Gravity
Molecular Weight 149.23 Partition Coefficient Not available.
Boiling Point 122 to 123°C @ 12mm Hg (251.6 to Vapor Pressure Not available.
253.4°F)
Melting Point Not available. Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Odor Not available.
Not available.
Not available. Not available.
Viscosity Taste
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability
Avoid excessive heat and light.
Incompatibilities
Reactive with strong oxidizing agents, strong acids.
Section XI. Toxicological Information
RTECS Number Not available.
Routes of Exposure Eye Contact. Ingestion. inhalation. Skin contact.
Not available.
Toxicity Data
CARCINOGENIC EFFECTS : Not available.
Chronic Toxic Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
Repeated or prolonged exposure to this compound is not known to aggravate existing medical conditions.
Acute Toxic Effects Irritating to eyes and skin on contact. Inhalation causes irritation of the lungs and respiratory system. Inflammation of the
eye is characterized by redness, watering, and itching. Skin inflammation is characterized by itching, scaling, reddening,
or, occasionally, blistering. Follow safe industrial hygiene practices and always wear proper protective equipment when
handling this compound.
Continued on Next Page
2-n-Butylaniline
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissove or mix material with a
Waste Disposal
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state and locl regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
Not applicable.
PIN Number
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification not available
(Canada)
EINECS Number (EEC) 220-272-6
EEC Risk Statements R36/37/38- Irritating to eyes, respiratory system and skin.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氨基-1-苯基-1-丁酮 1-(2-aminophenyl)butan-1-one 2034-40-4 C10H13NO 163.219 4-正丁基苯胺 4-Butylaniline 104-13-2 C10H15N 149.236 —— acetic acid-(2-butyl-anilide) 6971-78-4 C12H17NO 191.273 1-丁基-2-硝基苯 1-butyl-2-nitrobenzene 7137-55-5 C10H13NO2 179.219 丁苯 1-butylbenzene 104-51-8 C10H14 134.221 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-丁基苯肼 2-butylphenylhydrazine 58928-63-5 C10H16N2 164.25 —— 1,2-Bis(2-butylphenyl)diazene 61653-36-9 C20H26N2 294.44 —— 2-butyl-4’-(dimethylamino)azobenzene —— C18H23N3 281.401
反应信息
-
作为反应物:参考文献:名称:铁催化烷基芳基叠氮化物中C(sp3)-H键的分子内胺化摘要:亲核铁配合物Bu 4 N [Fe(CO)3(NO)](TBA [Fe])催化烷基芳基叠氮化物中未活化的C(sp 3)-H键的直接分子内胺化,导致形成取代的二氢吲哚和四氢喹啉衍生物。DOI:10.1002/anie.201704260
-
作为产物:参考文献:名称:Reilly; Hickinbottom, Journal of the Chemical Society, 1920, vol. 117, p. 133摘要:DOI:
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] HERBICIDAL PROPYNYL-PHENYL COMPOUNDS<br/>[FR] COMPOSÉS PROPYNYLE-PHÉNYLE HERBICIDES申请人:SYNGENTA PARTICIPATIONS AG公开号:WO2015197468A1公开(公告)日:2015-12-30The present invention relates to a compound of formula (I) wherein: R1 is C1-C3alkoxy, C1-C2alkoxy-C1-C3alkoxy, C1-C2fluoroalkoxy, ethyl, n-propyl, n-butyl, cyclopropyl or ethynyl; R2 is hydrogen, ethyl, n-propyl, cyclopropyl, vinyl, ethynyl, C1-C3alkoxy, C1-C3fluoroalkyl, C1-C2fluoroalkoxy, C1-C2alkoxy-C1-C3alkoxy-, or C1fluoroalkoxy-C1-C3alkoxy-; provided that when R1 is ethyl, n-propyl, n-butyl, cyclopropyl or ethynyl, then R2 is hydrogen, ethyl, n-propyl, cyclopropyl, vinyl or ethynyl; and Y is O, S, S(O), S(O)2, N(C1-C2alkyl), N(C1-C2alkoxy), C(O), CR8R9 or -CR10R11CR12R13-; and and G, R3, R4, R5 and R6 are as defined herein; wherein the compound of formula (I) is optionally present as an agrochemically acceptable salt thereof. These compounds are suitable for use as herbicides. The invention therefore also relates to a method of controlling weeds, especially grassy monocotyledonous weeds, in crops of useful plants, comprising applying a compound of formula (I), or a herbicidal composition comprising such a compound, to the plants or to the locus thereof.本发明涉及一种具有以下结构的化合物(I):其中:R1为C1-C3烷氧基,C1-C2烷氧基-C1-C3烷氧基,C1-C2氟烷氧基,乙基,正丙基,正丁基,环丙基或乙炔基;R2为氢,乙基,正丙基,环丙基,乙烯基,乙炔基,C1-C3烷氧基,C1-C3氟烷基,C1-C2氟烷氧基,C1-C2烷氧基-C1-C3烷氧基,或C1氟烷氧基-C1-C3烷氧基-;但是当R1为乙基,正丙基,正丁基,环丙基或乙炔基时,R2为氢,乙基,正丙基,环丙基,乙烯基或乙炔基;Y为O,S,S(O),S(O)2,N(C1-C2烷基),N(C1-C2烷氧基),C(O),CR8R9或-CR10R11CR12R13-;以及G,R3,R4,R5和R6如本文所定义;其中化合物(I)可作为农药中可接受的盐存在。这些化合物适用于用作除草剂。因此,本发明还涉及一种控制杂草,特别是草本单子叶杂草,在有用植物作物中的方法,包括将化合物(I)或含有这种化合物的除草剂组合物施用于植物或其生长地点。
-
Process for producing azasulfonium salts and rearrangement thereof to申请人:The Ohio State University Research Foundation公开号:US03985756A1公开(公告)日:1976-10-12Preparing ortho-substituted anilines by reacting an N-chloroaniline with a non-carbonylic di-hydrocarbon sulfide to form an azasulfonium chloride, reacting the azasulfonium chloride with a strong base to form an aniline substituted in the 2-position with a hydrocarbon-S-hydrocarbyl thio-ether group. The ortho-substituted thio-ether compounds can be reduced with a de-sulfurizing reducing agent such as Raney nickel or the like to form the ortho-alkylated aniline. The aniline may be an amino-pyridine. The azasulfonium salt and thio-ether intermediate products can be isolated and recovered. If desired, the thio-ether compounds can be reduced to form ortho-alkylated aniline products which are useful as intermediates for a wide variety of purposes, including their uses in making dyes, herbicides, and the like.
-
Process for preparation 2,6-dihydrobenzo[1:26, 4:5-6]-difuran dyes申请人:Imperial Chemical Industries PLC公开号:US05189181A1公开(公告)日:1993-02-23A process for the preparation of a polycyclic dye of the Formula (1): ##STR1## by reacting a substituted acetic acid of the Formula (2): ##STR2## with a compound of the Formula (3): ##STR3## and oxidation of the intermediate leuco compound to dehydrogenate the peripheral heterocyclic rings wherein Y is an optionally substituted aromatic or heteroaromatic radical; Ring A is unsubstituted or is substituted by from one to five groups; Z is --NR.sup.1 R.sup.2 ; R.sup.1 and R.sup.2 are each independently H or are independently selected from optionally substituted alkyl, alkenyl, cycloalkyl, aralkyl, aryl and heteroaryl; or R.sup.1 and R.sup.2 together with the nitrogen atom to which they are attached form a heterocyclic ring; or R.sup.1 and R.sup.2 each independently together with the nitrogen to which they are attached and the adjacent carbon atom of Ring B form a heterocyclic ring; and X.sup.1 and X.sup.2 are each independently selected from H, halogen, optionally substituted alkyl, optionally substituted aryl, optionally substituted heteroaryl, cyano, carbamoyl, sulphamoyl, carboxylic acid and carboxylic acid ester, a process for the preparation of the substituted acetic acid of Formula (2) and certain novel substituted acetic acids of Formula (2).一种制备式(1)多环染料的方法:通过使式(2)取代的乙酸与式(3)化合物反应,并将中间体隐色化合物氧化以脱氢边缘杂环,其中Y是可选地取代的芳香族或杂芳香族基团;环A是不取代的或由一个到五个组取代的;Z是-NR1R2;R1和R2各自独立为H或各自独立选自可选取代的烷基、烯基、环烷基、芳烷基、芳基和杂芳基;或者R1和R2与它们所连接的氮原子一起形成一个杂环;或者R1和R2各自独立地与它们所连接的氮和环B的相邻碳原子形成一个杂环;X1和X2各自独立选自H、卤素、可选取代的烷基、可选取代的芳基、可选取代的杂芳基、腈、碳酸酯、磺酰胺、羧酸和羧酸酯,一种制备式(2)取代乙酸的方法和某些新型的式(2)取代乙酸。
-
Palladium-Catalyzed Secondary Benzylic Imidoylative Reactions作者:Chenglong Wang、Licheng Wu、Wentao Xu、Feng He、Jingping Qu、Yifeng ChenDOI:10.1021/acs.orglett.0c02515日期:2020.9.4Reported herein is a palladium-catalyzed secondary benzylic imidoylative Negishi reaction leveraging the sterically bulky aromatic isocyanides as the imine source. This method allows the facile access of alkyl-, (hetero)aryl-, and alkynylzinc reagents to afford various α-substituted phenylacetone products under mild acidic hydrolysis, which are ubiquitous motifs in many pharmaceuticals and biologically
表征谱图
-
氢谱1HNMR
-
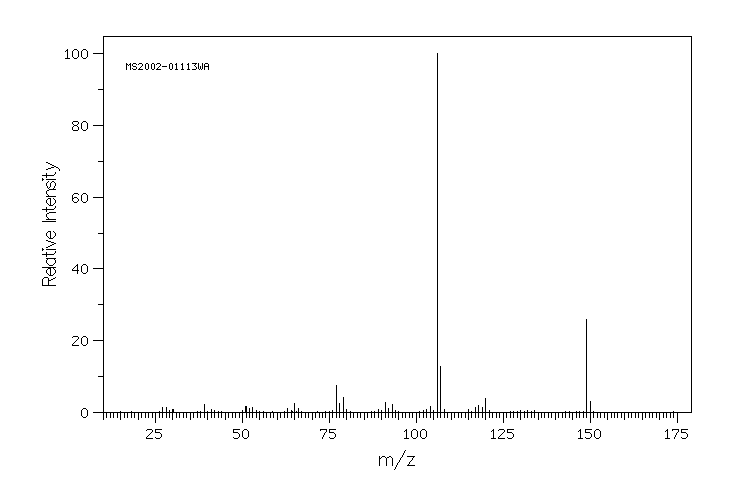
质谱MS
-
碳谱13CNMR
-
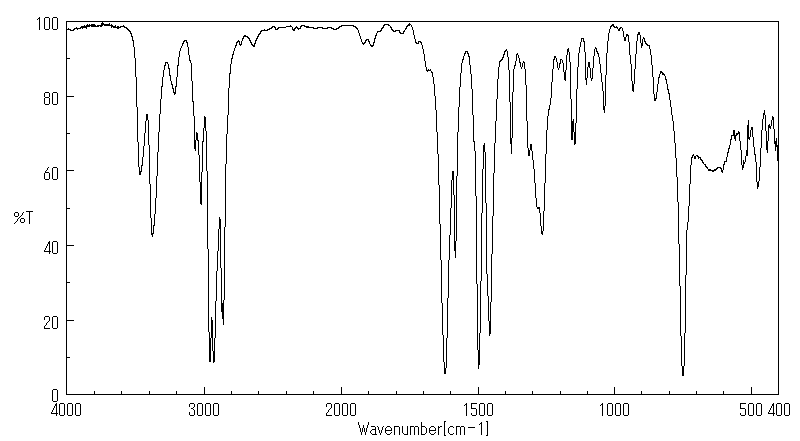
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫