氯乙酸异丙酯 | 105-48-6
中文名称
氯乙酸异丙酯
中文别名
氯醋酸异丙酯
英文名称
isopropyl chloroacetate
英文别名
isopropyl 2-chloroacetate;chloroacetic acid,1-methylethyl ester;propan-2-yl 2-chloroacetate
CAS
105-48-6
化学式
C5H9ClO2
mdl
MFCD00040410
分子量
136.578
InChiKey
VODRWDBLLGYRJT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:149-150 °C(lit.)
-
密度:1.096 g/mL at 25 °C(lit.)
-
闪点:159 °F
-
物理描述:Isopropyl chloroacetate appears as a clear colorless liquid. Less dense than water. Vapors heavier than air. Used to make other chemicals.
-
蒸汽压力:4.10 mmHg
-
保留指数:845;845;850.7;861;891
-
稳定性/保质期:
避免与氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:8
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:T
-
安全说明:S26,S37/39,S45
-
危险类别码:R10,R36/37/38,R25
-
WGK Germany:1
-
海关编码:2915400090
-
危险品运输编号:UN 2947 3/PG 3
-
危险类别:3
-
包装等级:III
-
储存条件:储存于阴凉、通风的库房,远离火种、热源,并防止静电。应与氧化剂、强碱分开存放,切忌混储。配备相应种类和数量的消防器材。储区应备有泄漏应急处理设备及合适的收容材料。
SDS
| Name: | Isopropyl Chloroacetate 99% Material Safety Data Sheet |
| Synonym: | (1-Methylethyl)-2-Chloroacetate |
| CAS: | 105-48-6 |
Synonym:(1-Methylethyl)-2-Chloroacetate
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 105-48-6 | Isopropyl Chloroacetate | 99% | 203-301-7 |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid if irritation develops or persists. Wash clothing before reuse. Flush skin with plenty of soap and water.
Ingestion:
Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. Get medical aid if cough or other symptoms appear. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 105-48-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: 68 mm Hg @20C
Viscosity: Not available.
Boiling Point: 149 - 150 deg C @ 760.00mm Hg
Freezing/Melting Point: -112 deg C
Autoignition Temperature: Not available.
Flash Point: 56 deg C ( 132.80 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: slightly soluble in water
Specific Gravity/Density: 1.0850g/cm3
Molecular Formula: C5H9ClO2
Molecular Weight: 136.57
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: May occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 105-48-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Isopropyl Chloroacetate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ISOPROPYL CHLOROACETATE
Hazard Class: 3
UN Number: 2947
Packing Group: III
IMO
Shipping Name: ISOPROPYL CHLOROACETATE
Hazard Class: 3.3
UN Number: 2947
Packing Group: III
RID/ADR
Shipping Name: ISOPROPYL CHLOROACETATE
Hazard Class: 3
UN Number: 2947
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 33 Take precautionary measures against static
discharges.
S 37/39 Wear suitable gloves and eye/face
protection.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 105-48-6: No information available.
Canada
CAS# 105-48-6 is listed on Canada's NDSL List.
CAS# 105-48-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 105-48-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:氯乙酸异丙酯 以 N,N-二甲基甲酰胺 、 异丙醇 为溶剂, 反应 6.05h, 生成 Propan-2-yl 2-[(2-oxo-2-propan-2-yloxyethyl)sulfamoyl]benzoate参考文献:名称:Schapira, Celia B.; Perillo, Isabel A.; Lamdan, Samuel, Journal of Heterocyclic Chemistry, 1980, vol. 17, p. 1281 - 1288摘要:DOI:
-
作为产物:参考文献:名称:用于热不稳定化合物的气相色谱/质谱分析以及重氮烷基乙酸烷基酯合成副产物的重新评估的简单准则。摘要:理由气相色谱(GC)和气相色谱/质谱(GC / MS)的主要限制是分析物的热不稳定性。我们建议,进样器和色谱柱的温度不应超过被分析系列的最高同系物的大气压沸点(无分解),而不是使用不同温度获得色谱图的耗时过程。方法选择了一系列热不稳定的重氮羰基化合物,重氮乙酸烷基酯(预测的稳定性极限为约140°C,重氮乙酸乙酯的沸点),使用标准设备进行GC / MS分析。选择不同的GC分离条件,以使目标化合物的保留温度均低于和高于140°C。结果分析重氮乙酸烷基酯在其热稳定性范围内可以重新分析其典型的合成副产物。没有发现富马酸二烷基酯或马来酸酯杂质,这是以前经常报道的主要分解产物。相反,乙醇酸硝酸盐的烷基酯O(2)NOCH(2)CO(2)R和“假二聚体”产品ROCO [C(2)H(3)NO] CO(2)R第一次发现。结论要避免在GC和/或GC / MS分析过程中热不稳定的有机化合物分解,需要估计其降解温度DOI:10.1002/rcm.6457
-
作为试剂:参考文献:名称:Difluoromethylation of some C–H acids with chlorodifluoromethane under conditions of phase transfer catalysis (PTC)摘要:Selected C-H acids react with difluorocarbene generated from chlorodifluoromethane with concentrated aqueous solution of sodium hydroxide, and a catalyst, benzyltriethylammonium chloride (TEBAC) in benzene or THF affording C-difluoromethyl substituted derivatives. This process is restricted to C-H acids of pK(a) congruent to 16.3-19.1. The observed facts are rationalized. (C) 2009 Elsevier B.V. All rights reserved.DOI:10.1016/j.jfluchem.2009.02.008
文献信息
-
Efficient Dimerization Disruption of <i>Leishmania infantum</i> Trypanothione Reductase by Triazole-phenyl-thiazoles作者:Alejandro Revuelto、Héctor de Lucio、Juan Carlos García-Soriano、Pedro A. Sánchez-Murcia、Federico Gago、Antonio Jiménez-Ruiz、María-José Camarasa、Sonsoles VelázquezDOI:10.1021/acs.jmedchem.1c00206日期:2021.5.13chemotype that yields noncompetitive, slow-binding inhibitors of LiTryR. Several compounds bearing (poly)aromatic substituents dramatically improve the ability to disrupt LiTryR dimerization relative to reference imidazoles. Molecular modeling studies identified an almost unexplored hydrophobic region at the interfacial domain as the putative binding site for these compounds. A subsequent structure-based通过破坏其同二聚体界面来抑制婴儿利什曼原虫锥硫酮二硫化物还原酶(Li TryR)已被证明是寻找新型抗利什曼原虫药物的替代且尚未开发的策略。概念证明首先是通过肽和肽模拟物获得的。基于之前报道的含有咪唑-苯基-噻唑支架的二聚化干扰剂,我们现在报道了一种新的基于 1,2,3-三唑的化学型,该化学型产生非竞争性、缓慢结合的Li TryR 抑制剂。相对于参考咪唑,几种带有(多)芳香族取代基的化合物显着提高了破坏Li TryR 二聚化的能力。分子建模研究确定了界面域中几乎未被探索的疏水区域作为这些化合物的假定结合位点。随后基于结构的设计产生了一种对称的三唑类似物,它表现出比Li TryR 更有效的抑制活性,并增强了杀利什曼病的活性。值得注意的是,其中几种新型含三唑化合物能够杀死细胞培养物中的细胞外和细胞内寄生虫。
-
AMIDINE COMPOUND OR SALT THEREOF申请人:Tanikawa Tetsuya公开号:US20140155597A1公开(公告)日:2014-06-05The purpose of the present invention is to provide a novel compound which has an anti-fungal activity on pathogenic fungi including fungi belonging to the genus Candida , the genus Aspergillus and the genus Trichophyton and is useful as a medicinal agent. A compound represented by formula (I) (wherein A 1 represents a nitrogen atom or a group represented by formula CR 6 ; A 2 and A 3 are the same as or different from each other and independently represent a nitrogen atom or a group represented by formula CH; R 1 represents an aryl group which may be substituted by 1 to 5 substituents independently selected from a substituent group (2) or the like; R 2 and R 3 are the same as or different from each other and independently represent a hydrogen atom, a halogen atom, a C 1-6 alkyl group, a C 1-6 haloalkyl group or a C 1-6 alkoxy group; and R 4 and R 5 are the same as or different from each other and independently represent a hydrogen atom, a C 1-6 haloalkyl group, a C 1-6 alkyl group or the like) or a salt thereof is useful as an anti-fungal agent.本发明的目的是提供一种新颖的化合物,该化合物对包括属于念珠菌属、曲霉属和毛癣菌属的病原真菌具有抗真菌活性,并可用作药物。由公式(I) (其中A 1代表氮原子或由公式CR 6表示的基团;A 2和A 3相同或不同,独立地表示氮原子或由公式CH表示的基团;R 1表示可能由1至5个独立选自取代基组(2)的取代基取代的芳基;R 2和R 3相同或不同,独立地表示氢原子、卤素原子、C 1-6烷基、C 1-6卤代烷基或C 1-6烷氧基;R 4和R 5相同或不同,独立地表示氢原子、C 1-6卤代烷基、C 1-6烷基等)或其盐用作抗真菌剂。
-
POLYCYCLIC PYRAZOLINONE DERIVATIVE AND HERBICIDE COMPRISING SAME AS EFFECTIVE COMPONENT THEREOF申请人:SAGAMI CHEMICAL RESEARCH INSTITUTE公开号:US20160024110A1公开(公告)日:2016-01-28Provided are a polycyclic pyrazolinone derivative indicated by general formula (1) (in the formula, R 1 , X 1 , X 2 , X 3 , and Y indicate the definitions provided in the Specification) and a herbicide comprising same as effective component thereof.
-
Synthesis and Biological Evaluation of a 5-6-5 Imidazole-Phenyl-Thiazole Based α-Helix Mimetic作者:Christopher G. Cummings、Nathan T. Ross、William P. Katt、Andrew D. HamiltonDOI:10.1021/ol8022962日期:2009.1.1that disrupt protein−protein interactions is a key goal in addressing a number of disease states. The α-helix is commonly found at protein interaction interfaces and has been the focus of substantial small molecule mimetic efforts. One of the primary drawbacks of many small molecule α-helix mimetics is their hydrophobic core structures. To address this problem we have developed a novel scaffold based
-
2-thienylglycidic derivative, process for its preparation and its use as申请人:Sanofi公开号:US05132435A1公开(公告)日:1992-07-21The invention relates to isopropyl 2-thienylglycidate of formula: ##STR1## obtained by reaction of 2-thienylcarboxaldehyde with an isopropyl haloacetate. It is employed as a synthesis intermediate in the preparation of 2-thienylacetaldoxime and of medications derived from thieno[3,2-c]pyridine.
表征谱图
-
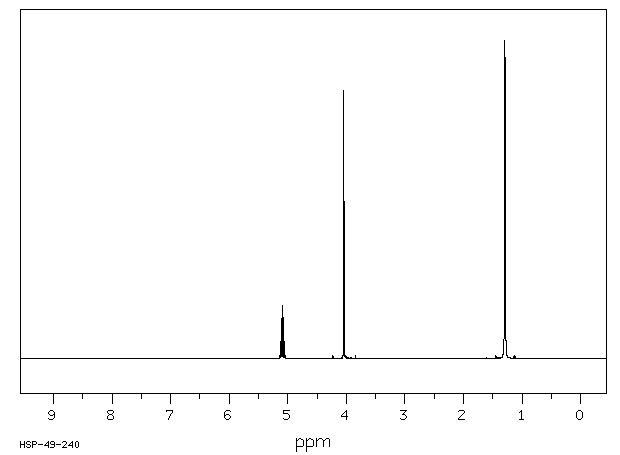
氢谱1HNMR
-
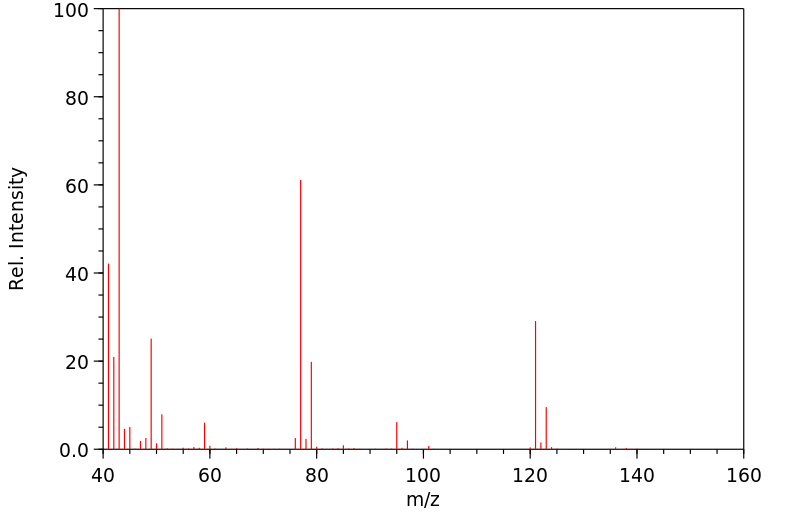
质谱MS
-
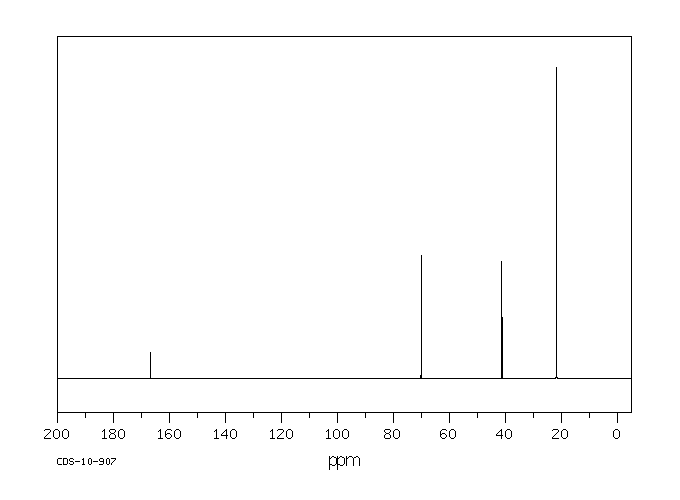
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸