N-苯甲基-N-苯肼盐酸盐 | 5705-15-7
中文名称
N-苯甲基-N-苯肼盐酸盐
中文别名
N-苄基-N-苯肼盐酸盐;1-苄基-1-苯肼盐酸盐
英文名称
N-benzyl-N-phenylhydrazine hydrochloride
英文别名
1-benzyl-1-phenylhydrazine hydrochloride;N-benzylphenylhydrazine hydrochloride;N′-benzyl-N′-phenylhydrazine hydrochloride;1-Benzyl-1-phenylhydrazine;hydron;chloride;1-benzyl-1-phenylhydrazine;hydron;chloride
CAS
5705-15-7
化学式
C13H14N2*ClH
mdl
MFCD00050690
分子量
234.728
InChiKey
JTYLHYOCBGPMNO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:178-181°C
-
稳定性/保质期:
在常温常压下,该物质稳定存在且呈现针状结晶。其熔点为170℃。
计算性质
-
辛醇/水分配系数(LogP):3.14
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.076
-
拓扑面积:29.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
TSCA:Yes
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
RTECS号:MU9800000
-
海关编码:2928000090
-
WGK Germany:3
SDS
1-Benzyl-1-phenylhydrazine Hydrochloride Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1-Benzyl-1-phenylhydrazine Hydrochloride
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1-Benzyl-1-phenylhydrazine Hydrochloride
Percent: >98.0%(T)
CAS Number: 5705-15-7
Chemical Formula: C13H14N2·HCl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
1-Benzyl-1-phenylhydrazine Hydrochloride
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Very pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:176 °C
Boiling Point/Range: No data available
1-Benzyl-1-phenylhydrazine Hydrochloride
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: ipr-rat LD50:404 mg/kg
ivn-mus LD50:106 mg/kg
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: MU9800000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1-Benzyl-1-phenylhydrazine Hydrochloride
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 1-Benzyl-1-phenylhydrazine Hydrochloride
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Category 2
Skin corrosion/irritation
Serious eye damage/eye irritation Category 2A
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements
Pictograms or hazard symbols
Signal word Warning
Hazard statement Causes skin irritation
Causes serious eye irritation
Precautionary statements
[Prevention] Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
[Response] IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Component(s): 1-Benzyl-1-phenylhydrazine Hydrochloride
Percent: >98.0%(T)
CAS Number: 5705-15-7
Chemical Formula: C13H14N2·HCl
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
1-Benzyl-1-phenylhydrazine Hydrochloride
Section 4. FIRST AID MEASURES
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Storage
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Law is followed.
Packaging material:
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
Color: White - Very pale yellow
Odor: No data available
pH: No data available
Melting point/freezing point:176 °C
Boiling Point/Range: No data available
1-Benzyl-1-phenylhydrazine Hydrochloride
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Hydrogen chloride
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: ipr-rat LD50:404 mg/kg
ivn-mus LD50:106 mg/kg
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
No data available
Reproductive toxicity:
RTECS Number: MU9800000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
1-Benzyl-1-phenylhydrazine Hydrochloride
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
有机合成。
用途简介 用途有机合成。
反应信息
-
作为反应物:描述:N-苯甲基-N-苯肼盐酸盐 在 4-二甲氨基吡啶 、 aluminum (III) chloride 作用下, 以 四氢呋喃 、 甲醇 、 苯 为溶剂, 反应 0.5h, 生成 1-tert-butyl 3-methyl 2-(2-methoxy-2-oxoethyl)-1H-indole-1,3-dicarboxylate参考文献:名称:Synthetic approaches toward the marine alkaloid prenostodione摘要:An efficient synthesis of the core of prenostodione (3) is described herein featuring the base condensation of BOC-protected indole diesters 21 and 24 with p-methoxybenzaldehyde (22) and 4-[(t-butyldimethylsilyl)oxy]benzaldehyde (26). Attempts at selective saponification of the resultant diesters yielded isoprenostodione (3a) bearing the ester functionality at the C-3 position of the indole ring. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2013.02.116
-
作为产物:描述:参考文献:名称:KATRITZKY, ALAN R.;RAO, M. SURESH CHANDER, J. CHEM. SOC. PERKIN TRANS. PT 1,(1989) N2, C. 2297-2303摘要:DOI:
-
作为试剂:描述:1-(2-tetrahydrothiopyranylthio)-3-(1-benzyl-3-indolyl)propane 在 盐酸 、 N-苯甲基-N-苯肼盐酸盐 、 溶剂黄146 作用下, 反应 8.0h, 以49%的产率得到3-(1-benzyl-3-indolyl)propanethiol参考文献:名称:Reaction of 3,4-dihydro-2H-thiopyran with phenylhydrazines. Synthesis of homothiotryptophols摘要:DOI:10.1007/bf00470691
文献信息
-
Asymmetric Synthesis of Tetracyclic Pyrroloindolines and Constrained Tryptamines by a Switchable Cascade Reaction作者:Corien de Graaff、Lisa Bensch、Sjoerd J. Boersma、Răzvan C. Cioc、Matthijs J. van Lint、Elwin Janssen、Nicholas J. Turner、Romano V. A. Orru、Eelco RuijterDOI:10.1002/anie.201507041日期:2015.11.16The interrupted Fischer indole synthesis of arylhydrazines and biocatalytically generated chiral bicyclic imines selectively affords either tetracyclic pyrroloindolines or tricyclic tryptamine analogues depending on the reaction conditions. We demonstrate that the reaction is compatible with a variety of functional groups. The products are obtained in high optical purity and in reasonable to good yield
-
Reduction of hydrazines to amines with aqueous solution of titanium(iii) trichloride作者:Yan Zhang、Qiang Tang、Meiming LuoDOI:10.1039/c1ob05328k日期:——cleavage in hydrazines is widely used in the preparation of amines and thus occupies a significant place in organic synthesis. In this paper, we report a new method for the reductive cleavage of N–N bonds in hydrazines by commercially available and cheap aqueous titanium(III) trichloride. The reaction proceeds smoothly under a broad pH range from acidic to neutral and basic conditions to afford amines in good
-
Leucine rich repeat kinase 2 (LRRK2) inhibitors based on indolinone scaffold: Potential pro-neurogenic agents作者:Irene G. Salado、Josefa Zaldivar-Diez、Víctor Sebastián-Pérez、Lingling Li、Larissa Geiger、Silvia González、Nuria E. Campillo、Carmen Gil、Aixa V. Morales、Daniel I. Perez、Ana MartinezDOI:10.1016/j.ejmech.2017.06.060日期:2017.9design, synthesis, biological evaluation, SAR and potential binding mode of indoline-like LRRK2 inhibitors and their preliminary neurogenic effect in neural precursor cells isolated from adult mice ventricular-subventricular zone. These results open new therapeutic horizons for the use of LRRK2 inhibitors as neuroregenerative agents. Moreover, the indolinone derivatives here prepared, inhibitors of the
-
Synthesis of new pyrrolo[3,4-b]indol-3-ones as latent substrates for pyrrolo[3,4-b]indoles作者:Mark G. Saulnier、Steven M. Schreiber、Kerri L. Cavanaugh、Robert B. Perni、H. Howard Joyner、and Gordon W. GribbleDOI:10.24820/ark.5550190.p011.365日期:——editorial office Abstract We report the synthesis of new examples of the 1,4-dihydropyrrolo[3,4-b]indol-3(2H)-one ring system via Fischer indolization between the appropriate phenylhydrazines and pyrrolidine-2,3-diones. Lithiation at the C1 position with lithium diisopropylamide following by quenching with iodomethane affords 1-methyl-1,4dihydropyrrolo[3,4-b]indol-3(2H)-ones in excellent yield.
-
An Improved Synthesis of β-Carboline and Azepino-and Azocino[3,4-b]Indole Derivatives from Lactams作者:G. P. TokmakovDOI:10.1007/s10593-014-1463-x日期:2014.5treatment with aryl hydrazines afforded the 2,3,4,9-tetrahydro-1Н-β-carbolin-1-ones, 3,4,5,10-tetrahydroazepino[3,4-b]indol-1(2Н)-ones, and 2,3,4,5,6,11-hexahydro-1Н-azocino[3,4-b]indol-1-ones. The synthesis was performed as a one-pot process without isolating the intermediate α-formyl lactams.
表征谱图
-
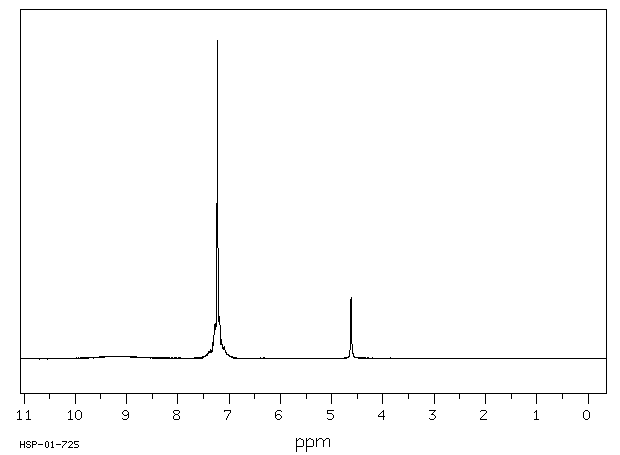
氢谱1HNMR
-
质谱MS
-
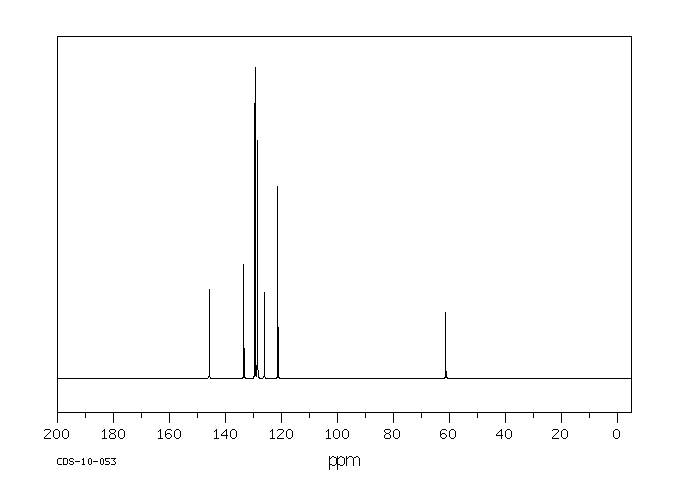
碳谱13CNMR
-
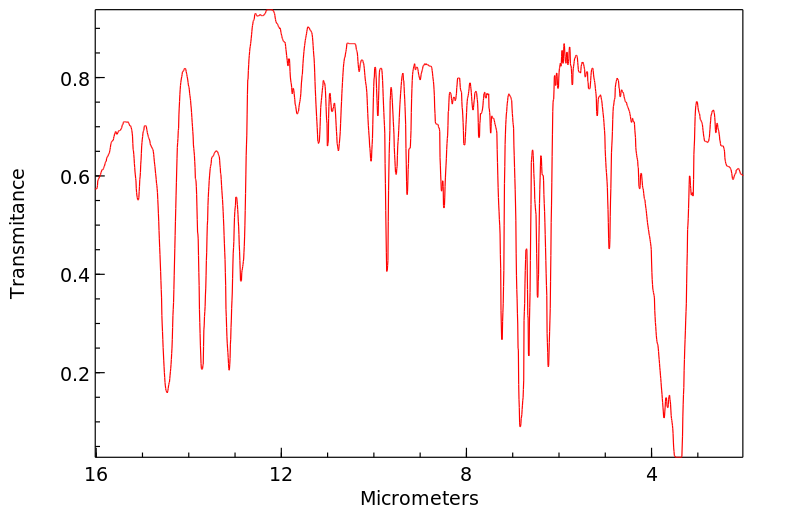
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫