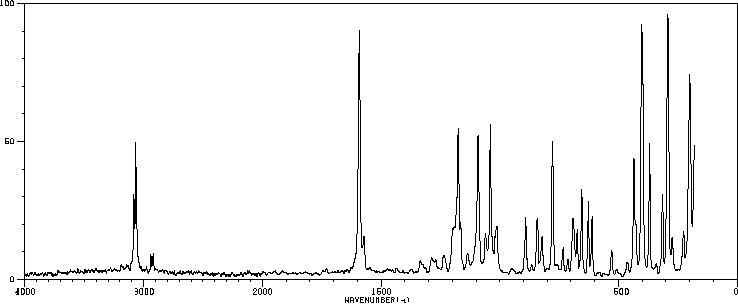
代谢
DDT在胃和肠道被吸收后,进入淋巴系统,随后在全身传播并融入脂肪组织。DDT的代谢主要通过肝脏和肾脏中的细胞色素P-450酶进行,在那里它经历还原脱氯作用,转化为DDD(二氯二苯基二氯乙烷)和DDE(二氯二苯基二氯乙烯)。这些化合物进一步分解成更多的代谢物,主要是DDA(双(对氯苯基)醋酸),它们通过尿液排出体外。
DDT is absorbed in the stomach and intestine, after which it enters the lymphatic system and is carried throughout the body and incorporated into fatty tissues. Metabolism of DDT occurs mainly via cytochrome P-450 enzymes in the liver and kidney, where it undergoes reductive dechlorination to DDD (dichlorodiphenyldichloroethane) and DDE (dichlorodiphenyldichloroethylene). These compounds are further degraded into additional metabolites, mainly DDA (bis(p-chlorophenyl) acetic acid), which are excreted in the urine. (L85)
来源:Toxin and Toxin Target Database (T3DB)