2,4'-二氯二苯甲酮 | 85-29-0
中文名称
2,4'-二氯二苯甲酮
中文别名
2,4'-二氯二苯酮;二氯苯甲酮;2,4-二氯苯甲酮;2,4'-二氯苯甲酮;2,4"-二氯二苯甲酮;2,4-二氯二苯甲酮
英文名称
2,4'-dichlorobenzophenone
英文别名
(2-chlorophenyl)(4-chlorophenyl)methanone;2,4'-Dichlor-benzophenon;o,p'-dichlorobenzophenone;2,4’-dichlorobenzophenone;(2-chlorophenyl)-(4-chlorophenyl)methanone
CAS
85-29-0
化学式
C13H8Cl2O
mdl
MFCD00038744
分子量
251.112
InChiKey
YXMYPHLWXBXNFF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64°C
-
沸点:214 °C / 22mmHg
-
密度:1.3930
-
溶解度:可溶于氯仿、甲醇(微量)
-
保留指数:1890.4
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:16
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
安全信息
-
TSCA:Yes
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2914700090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温和干燥环境下使用。
SDS
2,4'-Dichlorobenzophenone Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,4'-Dichlorobenzophenone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,4'-Dichlorobenzophenone
Percent: >99.0%(GC)
CAS Number: 85-29-0
Chemical Formula: C13H8Cl2O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,4'-Dichlorobenzophenone
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:64°C
214°C/2.9kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2,4'-Dichlorobenzophenone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,4'-Dichlorobenzophenone
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,4'-Dichlorobenzophenone
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,4'-Dichlorobenzophenone
Percent: >99.0%(GC)
CAS Number: 85-29-0
Chemical Formula: C13H8Cl2O
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
2,4'-Dichlorobenzophenone
Section 5. FIRE-FIGHTING MEASURES
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Very pale yellow
Odour: No data available
pH: No data available
Melting point/freezing point:64°C
214°C/2.9kPa
Boiling point/range:
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
No data available
[Water]
[Other solvents] No data available
2,4'-Dichlorobenzophenone
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen chloride
products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
Skin corrosion/irritation: No data available
No data available
Serious eye
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
No data available
Reproductive toxicity:
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
2,4'-Dichlorobenzophenone
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4,4'-二氯二苯甲酮 4,4'-Dichlorobenzophenone 90-98-2 C13H8Cl2O 251.112 —— 2,4'-dichlorodiphenylmethane 52094-02-7 C13H10Cl2 237.128 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-氯-4'-氟二苯甲酮 (2-chlorophenyl)(4-fluorophenyl)methanone 1806-23-1 C13H8ClFO 234.657 —— 5,3'-diamino-2,4'-dichloro-benzophenone 96662-54-3 C13H10Cl2N2O 281.141 4,4'-二氯二苯甲酮 4,4'-Dichlorobenzophenone 90-98-2 C13H8Cl2O 251.112 —— 2,4'-dichlorodiphenylmethane 52094-02-7 C13H10Cl2 237.128 —— (2-chloro-phenyl)-bis-(4-chloro-phenyl)-methane 93694-04-3 C19H13Cl3 347.671 1-氯-2-[1-(4-氯苯基)乙基]苯 1-(o-chlorophenyl)-1-(p-chlorophenyl)ethane 77008-62-9 C14H12Cl2 251.155 1-氯-2-[1-(4-氯苯基)乙烯基]苯 1-o-Chlorophenyl-1-p'-chlorophenylethylene 39274-24-3 C14H10Cl2 249.139 1-[溴-(2-氯苯基)甲基]-4-氯苯 2,4'-dichlorodiphenyl bromomethane 61023-86-7 C13H9BrCl2 316.024 (2-氯苯基)-(4-氯苯基)甲醇 (2-chlorophenyl)(4'-chlorophenyl)methanol 43171-49-9 C13H10Cl2O 253.128 —— (R)-(4-chlorophenyl)(2-chlorophenyl)methanol 148259-79-4 C13H10Cl2O 253.128
反应信息
-
作为反应物:描述:参考文献:名称:1,1-Dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane and Related Compounds1摘要:DOI:10.1021/jo01059a110
-
作为产物:描述:参考文献:名称:The Chemical Composition of Technical DDT1摘要:DOI:10.1021/ja01225a058
文献信息
-
Preparation of Functionalized Diaryl- and Diheteroaryllanthanum Reagents by Fast Halogen-Lanthanum Exchange作者:Andreas D. Benischke、Lucile Anthore-Dalion、Guillaume Berionni、Paul KnochelDOI:10.1002/anie.201709553日期:2017.12.18Aryl and heteroaryl halides (X=Br, I) undergo a fast and convenient halogen–lanthanum exchange with nBu2LaMe, which leads to functionalized diaryl‐ and diheteroaryllanthanum derivatives. Subsequent trapping reactions with selected electrophiles, such as ketones, aldehydes, or amides, proceeded smoothly at −50 °C in THF, affording polyfunctionalized alcohols and carbonyl derivatives. Kinetic competition
-
MgCl2-Accelerated Addition of Functionalized Organozinc Reagents to Aldehydes, Ketones, and Carbon Dioxide作者:Albrecht Metzger、Sebastian Bernhardt、Georg Manolikakes、Paul KnochelDOI:10.1002/anie.201000634日期:2010.6.21Pump it up! The sluggish reactivity of organozinc reagents in additions to aldehydes, ketones, and CO2 can be increased by MgCl2, which is usually generated in the preparation of the zinc reagent. The direct reaction with CO2, in particular, opens an expeditious route to phenylacetic acid derivatives, as demonstrated in a short synthesis of ibuprofen (see scheme).
-
Chiral N-heterocyclic carbene iridium catalyst for the enantioselective hydrosilane reduction of ketones作者:Yoshiki Manabe、Kanako Shinohara、Hanako Nakamura、Hiro Teramoto、Satoshi SakaguchiDOI:10.1016/j.molcata.2016.05.020日期:2016.9NHC–Ag complex derived from N -(1-naphthalenylmethyl)-substituted benzimidazolium salt L12 , affording the corresponding alcohol in 92% yield and with 92% ee. Moreover, the evaluation of an Ir catalyst precursor showed that cationic [Ir(cod) 2 ]BF 4 complex could be used. Furthermore, the introduction of a chiral hydroxyamide side arm into the benzimidazolium salt was critical for the successful design of
-
Direct Addition of Functionalized Organozinc Reagents to Carbon Dioxide, Ketones, and Aldehydes in the Presence of MgCl2作者:Paul Knochel、Sebastian Bernhardt、Albrecht MetzgerDOI:10.1055/s-0030-1258314日期:2010.11functionalized organozinc reagents undergo smooth addition reactions at ambient temperature to carbon dioxide, ketones, and aldehydes in the presence of stoichiometric amounts of MgCl2. Several reactions were performed on a 20 mmol scale. organozinc reagents - magnesium chloride - carbon dioxide - phenylacetic acid derivatives - addition reactions
-
[EN] AZETIDINECARBOXAMIDE DERIVATIVES AND THEIR USE IN THE TREATMENT OF CB1 RECEPTOR MEDIATED DISORDRS<br/>[FR] DERIVES AZETIDINECARBOXAMIDE ET LEUR UTILISATION POUR LE TRAITEMENT DE TROUBLES A MEDIATION DU RECEPTEUR DU CB1申请人:VERNALIS RES LTD公开号:WO2004096794A1公开(公告)日:2004-11-11Compounds of formula (I) and their use in therapy, particularly for the treatment of a disorder mediated by CB1 receptors, such as obesity, wherein: R1 is aryl or heteroaryl; R2 is alkyl, aryl or heteroaryl; R3 is alkyl, aryl, heteroaryl, NR9R10, OR 15, or NR 16C(O)R17; Y is C=O, C=S, SO2, or (CR7R8)p; m = 1 or 2; n = 1 or 2; and p=1,2,3 or 4, R7 to R17 being as defined in the specification; wherein if -Y-R3 is -C(O)NH(alkyl) then: R1 and/or R2 is selected from heteroary1; and/or m and/or n is 2; and/or R11 and/or R12 is lower alkyl, or a pharmaceutically acceptable salt or prodrug thereof.化合物的化学式(I)及其在治疗中的应用,特别是用于治疗由CB1受体介导的疾病,如肥胖,其中:R1为芳基或杂环芳基;R2为烷基,芳基或杂环芳基;R3为烷基,芳基,杂环芳基,NR9R10,OR 15,或NR 16C(O)R17;Y为C=O,C=S,SO2,或(CR7R8)p;m = 1或2;n = 1或2;p=1,2,3或4,R7到R17如规范中定义;其中如果-Y-R3为-C(O)NH(烷基)则:R1和/或R2从杂芳基中选择;和/或m和/或n为2;和/或R11和/或R12为较低烷基,或其药用盐或前药。
表征谱图
-
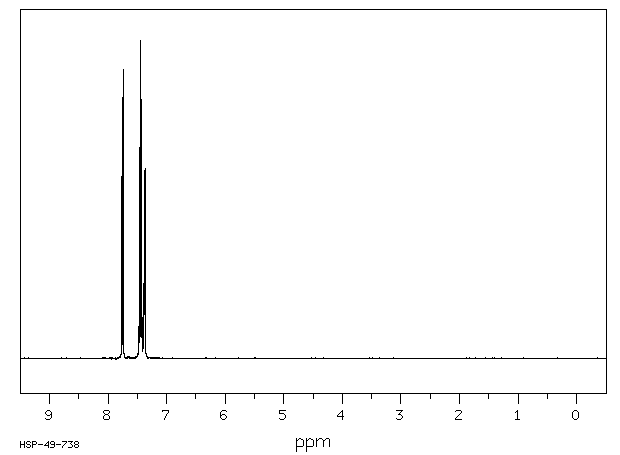
氢谱1HNMR
-
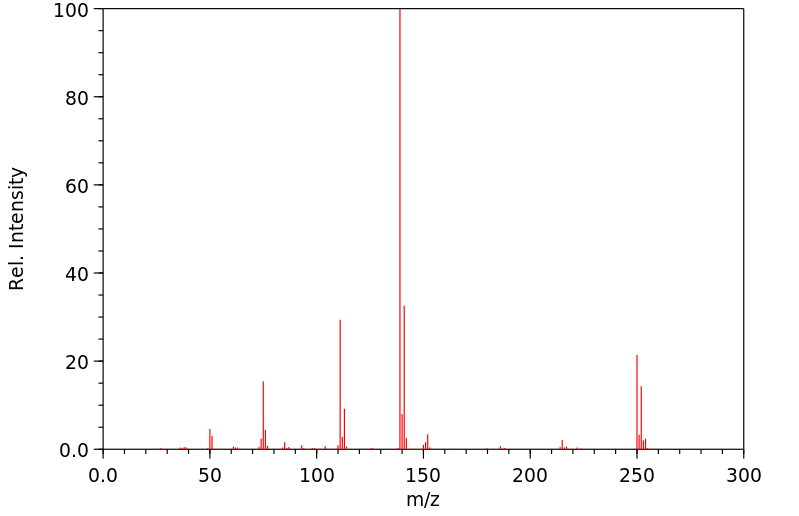
质谱MS
-
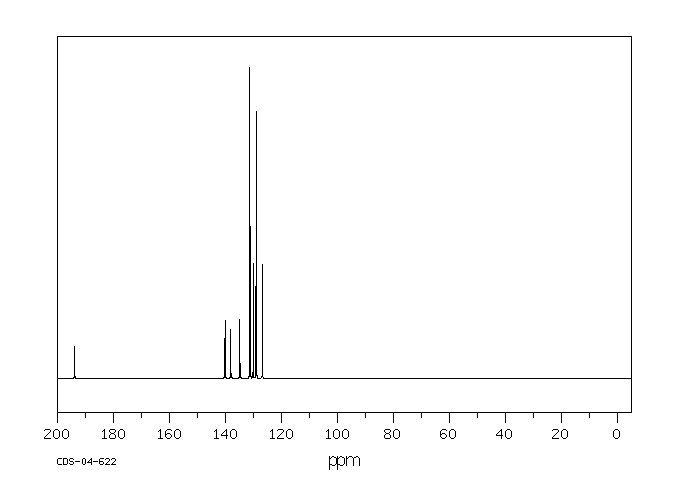
碳谱13CNMR
-
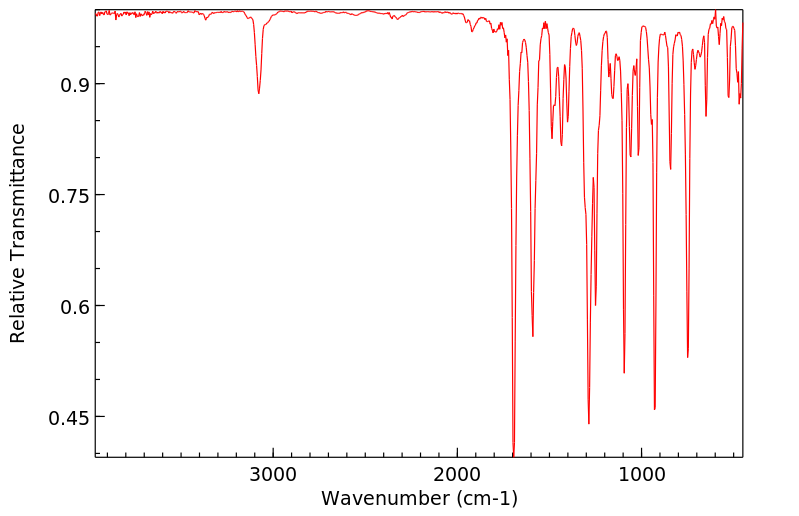
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫