萜品 | 80-53-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:35-40 °C
-
沸点:213-218 °C(lit.)
-
密度:0.934 g/mL at 20 °C(lit.)
-
闪点:87℃ (Cleveland open test)
-
物理描述:Solid
-
溶解度:1 mg/mL at 20 °C
-
保留指数:1279
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
SDS
| Name: | Terpineol Material Safety Data Sheet |
| Synonym: | 3-cyclohexene-1-methanol; terpeno |
| CAS: | 8006-39-1 |
Synonym:3-cyclohexene-1-methanol; terpeno
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 8006-39-1 | Terpineol | 100 | 232-268-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Not available.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May cause kidney damage. May cause central nervous system depression, characterized by excitement, followed by headache, dizziness, drowsiness, and nausea. Advanced stages may cause collapse, unconsciousness, coma and possible death due to respiratory failure.
Inhalation:
May cause respiratory tract irritation. May cause effects similar to those described for ingestion.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 8006-39-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: 0.1 mm Hg @ 37.7C
Viscosity: Not available.
Boiling Point: 214-224C
Freezing/Melting Point: 31-35C
Autoignition Temperature: Not applicable.
Flash Point: 193 deg F ( 89.44 deg C)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: Very sightly soluble in water
Specific Gravity/Density: 0.93
Molecular Formula: C10H18O
Molecular Weight: 154.1254
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, acid chlorides, acid anhydrides.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 8006-39-1: WZ6600000 LD50/LC50:
CAS# 8006-39-1: Draize test, rabbit, skin: 500 mg/24H Moderate; Oral, rat: LD50 = 4300 mg/kg.
Carcinogenicity:
Terpineol - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
WGK (Water Danger/Protection)
CAS# 8006-39-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 8006-39-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 8006-39-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
松油醇是一种无色的稠厚液体,具有紫丁香花的独特香气。其凝固点为37℃,沸点为219℃。该物质易溶于乙醇、丙酮、氯仿、乙醚和苯等有机溶剂,而不溶于水。根据研究,人经口摄入的半数致死量(LD50)约为3~4g/kg,大白鼠则为4.3g/kg。按照卫生标准,其每日允许摄入量(ADI)为1mg/kg。
用途松油醇是我国批准使用的食用香料之一,主要用于柠檬、甜橙、草莓、桃等水果的香精中。在不同食品中的使用量有具体规定:口香糖40mg/kg;调味料38mg/kg;烘烤食品19mg/kg;冷饮16mg/kg;糖果14mg/kg;布丁类12~16mg/kg;软饮料5.4mg/kg。GB 2760—1996规定,松油醇可用于配制柠檬、白柠檬等柑橘类水果香精以及桃子、樱桃、树莓等香精。
生产方法松油醇可以从松树干经干馏或水蒸气蒸馏后分馏获得。另一种合成方法是用23%的稀硫酸处理松节油,使其中的α-蒎烯生成-水合萜二醇,再与磷酸共热蒸馏而成。此外,1,8-萜二醇脱水也可以制备松油醇。
工业上通常以松节油或异戊二烯为原料来制备。一种常见的硫酸催化两步法包括:在30%的硫酸作用下,松节油中的蒎烯生成水合萜二醇结晶沉淀,分离后,在0.2%的硫酸催化下脱水生成松油醇,分层分离得粗醇,经减压蒸馏得成品。相转移催化法则可以提高反应效果:将33g松节油、50g 80%硫酸和0.1g三乙基苄基氯化铵(TEBA)加入烧瓶,在12~14℃下快速搅拌反应12h,得到白色粉末状萜二醇;然后在102~105℃搅拌反应4~5小时后,静置除去水层。油层用3%的氢氧化钠溶液中和、静置分层得黄色油状液体,减压蒸馏收集190~200℃(90kPa)馏分,得到精品。另一种方法是异戊二烯与丁烯酮缩合后与格氏试剂反应制得松油醇。还有一种磷酸催化一步法:在70℃下强烈搅拌反应16小时后,冷却静置,分离出水层和油层。油层用稀NaOH溶液中和至中性,再用少量食盐水洗涤、干燥后减压蒸馏得到成品。
分类松油醇属于易燃液体,具有一定的毒性分级和急性毒性数据,以及刺激数据,并且在储存和运输时需要注意其可燃性和危险特性。具体的职业卫生标准包括STEL 5毫克/立方米。
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:一种一步法制备1,8-对孟烷二胺的方法摘要:本发明提供了一种一步法制备1,8‑对孟烷二胺的方法,该方法采用不饱和松节油单萜为反应原料,该方法还以强质子酸为反应原料,该方法还以含氮无机化合物作为反应原料,在水中进行一步反应,待反应完全后经萃取和减压蒸馏得到1,8‑对孟烷二胺纯品。本发明方法步骤少、易纯化,一步法直接制备1,8‑对孟烷二胺,产品纯度高,收率高,副产物为二氧化碳和硫酸盐,气体经吸收处理,硫酸盐能够直接处理。本发明首次采用不饱和松节油单萜、强质子酸和含氮无机化合物三者共同作为反应原料,才能实现一步法反应,克服了传统的制备方法中将质子酸作为催化剂使用的合成思路无法实现一步法反应的技术缺陷。公开号:CN113200872B
-
作为产物:参考文献:名称:Tiemann; Schmidt, Chemische Berichte, 1895, vol. 28, p. 1783摘要:DOI:
文献信息
-
ALPHA-AMINO ACID DERIVATIVES FOR IMPROVING SOLUBILITY
-
Formulation assistants申请人:Pflucker Frank公开号:US20070141014A1公开(公告)日:2007-06-21The invention relates to the use of compounds of the formula (I), where R 1 , R 2 and R 3 may be identical or different and are selected from: straight-chain or branched C 1 - to C 24 -alkyl groups, straight-chain or branched C 3 - to C 24 -alkenyl groups, straight-chain or branched C 1 - to C 24 -hydroxyalkyl groups, where the hydroxyl group may be bonded to a primary or secondary carbon atom of the chain and furthermore the alkyl chain may also be interrupted by oxygen, and/or C 3 - to C 10 -cycloalkyl groups and/or C 3 - to C 12 -cycloalkenyl groups, where the rings may in each case also be bridged by (CH 2 ) n groups, where n=1 to 3, as formulation assistants for the preparation of cosmetic or dermatological compositions, to corresponding novel compounds, and to the preparation thereof.该发明涉及使用式(I)的化合物,其中R1、R2和R3可能相同也可能不同,并且可从以下中选择:直链或支链的C1到C24烷基基团、直链或支链的C3到C24烯基基团、直链或支链的C1到C24羟基烷基基团,其中羟基可能与链的一级或二级碳原子结合,此外烷基链也可能被氧原子中断,和/或C3到C10环烷基基团和/或C3到C12环烯基基团,环可能在每种情况下也被(CH2)n基桥接,其中n=1至3,作为化妆品或皮肤科学组合物的制剂助剂,以及相应的新化合物的制备。
-
Ningalin b analogs employable for reversing multidrug resistance申请人:——公开号:US20030220320A1公开(公告)日:2003-11-27Anlogs of ningalin B lacking inherent cytotoxic activity may be employed to reverse multi-drug resistant (MDR) phenotype and to resensitize transformed cells, including a human colon cancer cell line (HCT116/VM46), with respect to a variety of cytotoxic agents, e.g., vinblastine and doxorubicin. In many instances, resensitization is achieved at lower doses than the prototypical agent verapamil. Total synthesis of ningalin B and its analogs was achieved using a concise, efficient approach based on a heterocyclic azadiene Diels-Alder strategy (1,2,4,5-tetrazine → 1,2-diazine → pyrrole) ideally suited for construction of the densely functionalized pyrrole core found in the natural product is detailed.
-
一种通过3-蒈烯制备柠檬烯的方法
-
Synthesis and Herbicidal Activities of <i>p</i>-Menth-3-en-1-amine and Its Schiff Base Derivatives作者:Shouji Zhu、Shichao Xu、Wang Jing、Zhendong Zhao、Jianxin JiangDOI:10.1021/acs.jafc.6b03977日期:2016.12.2813C NMR spectral analyses, and their pre-emergence herbicidal activities against ryegrass were evaluated. All of the compounds showed excellent herbicidal activity. The Schiff bases showed stronger herbicidal activities than the original amine 4. These compounds showed herbicidal activities comparable to that of glyphosate. The herbicidal activities of 5k and 5l against ryegrass shoot growth were 78
表征谱图
-
氢谱1HNMR
-
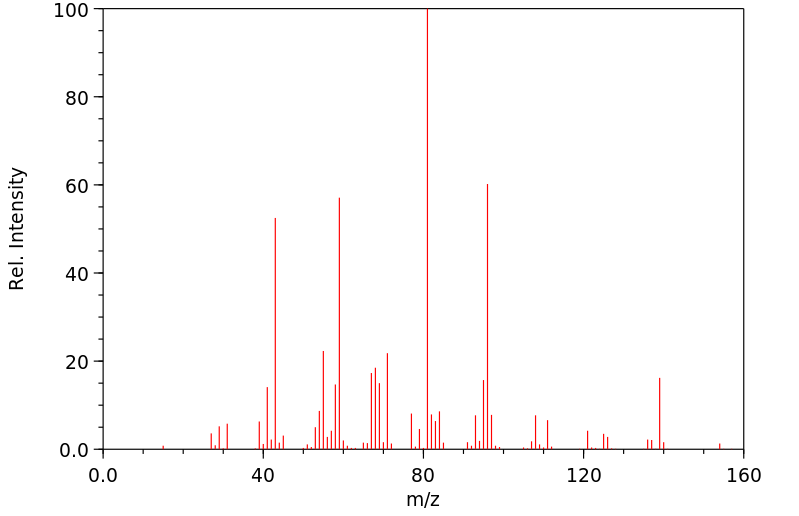
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息