乳清酸甲酯 | 6153-44-2
中文名称
乳清酸甲酯
中文别名
甲基乳清酸;2,6-二氧代-1,2,3,6-四氢嘧啶-4-甲酸甲酯
英文名称
methyl orotate
英文别名
orotic acid methyl ester;Methyl-orotat;6-carbomethoxy-uracil;methyl 2,4-dioxo-1H-pyrimidine-6-carboxylate
CAS
6153-44-2
化学式
C6H6N2O4
mdl
MFCD00010564
分子量
170.125
InChiKey
UUTDWTOZAWFKFW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:244 °C
-
沸点:299.73°C (rough estimate)
-
密度:1.5523 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):-0.9
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:84.5
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37,S39
-
危险类别码:R36/37/38
-
海关编码:2933599090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封储存,应存放在阴凉、干燥的仓库中。
SDS
| Name: | Methyl orotate 99% Material Safety Data Sheet |
| Synonym: | Orotic acid methyl ester; Methyl 1,2,3,6-tetrahydro-2,6-dioxopyrimidine-4-carboxylat |
| CAS: | 6153-44-2 |
Synonym:Orotic acid methyl ester; Methyl 1,2,3,6-tetrahydro-2,6-dioxopyrimidine-4-carboxylat
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6153-44-2 | Methyl orotate | 99 | 228-171-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6153-44-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 244 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: > 244 deg C
Solubility in water: Not available.
Specific Gravity/Density: Not available.
Molecular Formula: C6H6N2O4
Molecular Weight: 170.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Will not occur.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6153-44-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Methyl orotate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 6153-44-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6153-44-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6153-44-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二氧代-1,2,3,6-四氢-4-嘧啶羰基氯化物 orotic acid chloride 3346-64-3 C5H3ClN2O3 174.543 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-氯2,6-二羟基-4-嘧啶羧酸甲酯 5-chloro-2,4-dihydroxy-6-methoxycarboxylpyrimidine 91447-90-4 C6H5ClN2O4 204.57 —— 5-iodo methyl orotate 116393-71-6 C6H5IN2O4 296.021 6-(羟基甲基)尿嘧啶 6-(hydroxymethyl)pyrimidine-2,4(1H,3H)-dione 22126-44-9 C5H6N2O3 142.114 —— methyl 2,6-dioxo-5-(trifluoromethyl)-1,2,3,6-tetrahydropyrimidine-4-carboxylate 936476-63-0 C7H5F3N2O4 238.123 —— methyl 3-butyl-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylate 1072131-89-5 C10H14N2O4 226.232 2,6-二氧代-1,2,3,6-四氢-4-嘧啶羰基氯化物 orotic acid chloride 3346-64-3 C5H3ClN2O3 174.543 1,3-二甲基乳清酸 1,3-dimethylorotic acid 4116-38-5 C7H8N2O4 184.152 2,6-二氧代-1,2,3,6-四氢嘧啶-4-羧酰胺 Orotsaeure-amid 769-97-1 C5H5N3O3 155.113
反应信息
-
作为反应物:参考文献:名称:Efficient and regioselective synthesis of pyrimido[5,4-d]pyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraones with diversified substitutions摘要:A highly regioselective synthesis of N3-, N5-, and N7-substituted pyrimidopyrimidinetetraones is reported. The synthesis of N5-monosubstituted and N5-, N7-disubstituted compounds was achieved through selective alkylation and urea mediated pyrimidinedione formation reactions. For the more synthetically challenging N3-, N5-, and N7-trisubstituted structures, in combination with computational studies, a palladium catalyzed cascade reaction was successfully employed for the simultaneous formation of the pyrimidinedione ring and functionalization of the N3 position, which provided the trisubstituted products in high yields with full control of regioselectivity. By applying this methodology, key intermediate 26 was prepared in a few synthetic steps in good overall yield for further functionalization and quick SAR development. (C) 2012 Published by Elsevier Ltd.DOI:10.1016/j.tetlet.2012.10.109
-
作为产物:描述:参考文献:名称:Synthesis of Substituted Pyrimidine Hydrazine Acids (PHA) and Their Use in Peptide Recognition摘要:DOI:10.3987/com-05-s(t)6
文献信息
-
The [2+2] Photocycloaddition of Uracil Derivatives with Ethylene as a General Route to <i>cis</i>-Cyclobutane β-Amino Acids作者:David Aitken、Christine Gauzy、Bertrand Saby、Elisabeth Pereira、Sophie FaureDOI:10.1055/s-2006-941571日期:2006.6A three-step procedure, based on the [2+2]-photochemical reaction of uracils with ethylene followed by controlled degradation of the heterocyclic ring, has been developed for the synthesis of a range of C1- and C2-substituted cis-cyclobutane β-amino acids, in good overall yield.
-
Synthesis of New Polyamine Derivatives for Cancer Chemotherapeutic Studies作者:Louis T. Weinstock、William J. Rost、C.C. ChengDOI:10.1002/jps.2600700835日期:1981.8Selected homologs, analogs, and acylated derivatives of spermine and spermidine, together with several heterocyclic and aromatic compounds containing a novoldiamine side chain, were prepared and evaluated biologically. Several compounds possessed activity against B-16 melanoma and human epidermoid carcinoma of the nasopharynx.
-
[EN] PYRIMIDINE DERIVATIVES AND THEIR USE AS HERBICIDES<br/>[FR] DÉRIVÉS DE PYRIMIDINE ET LEUR UTILISATION EN TANT QU'HERBICIDES申请人:SYNGENTA LTD公开号:WO2010092339A1公开(公告)日:2010-08-19The present invention relates to substituted pyrimidine derivatives, as well as N- oxides thereof and agriculturally acceptable salts thereof, and their use to control undesired plant growth, in particular in crops of useful plants. The invention extends to herbicidal compositions comprising such compounds, N-oxides and/or salts as well as mixtures of the same with one or more further active ingredient (such as, for example, an herbicide, fungicide, insecticide and/or plant growth regulator) and/or a safener. The invention further relates to intermediates useful in the preparation of such compounds, and to processes for their preparation.
-
一种5-三氟甲基尿嘧啶的合成方法
-
2-SUBSTITUTED-6-AMINO-5-ALKYL, ALKENYL OR ALKYNYL-4-PYRIMIDINECARBOXYLIC ACIDS AND 6-SUBSTITUTED-4-AMINO-3- ALKYL, ALKENYL OR ALKYNYL PICOLINIC ACIDS AND THEIR USE AS HERBICIDES申请人:Epp Jeffrey B.公开号:US20090088322A1公开(公告)日:2009-04-026-Amino-4-pyrimidinecarboxylic acids having alkyl, alkenyl or alkynyl substituents in the 5-position and 4-aminopicolinic acids having alkyl, alkenyl or alkynyl substituents in the 3-position, and their amine and acid derivatives, are potent herbicides demonstrating a broad spectrum of weed control.
表征谱图
-
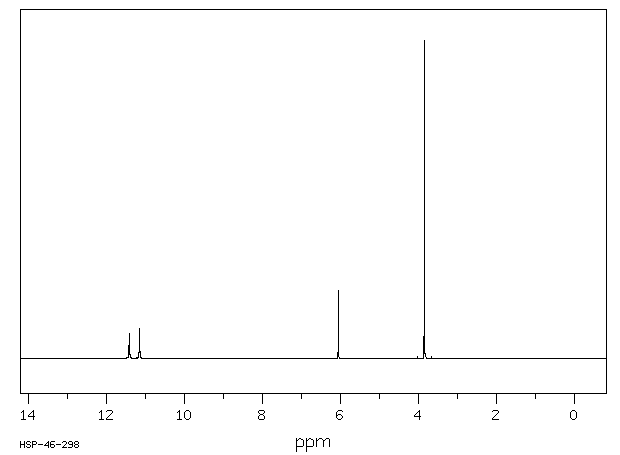
氢谱1HNMR
-
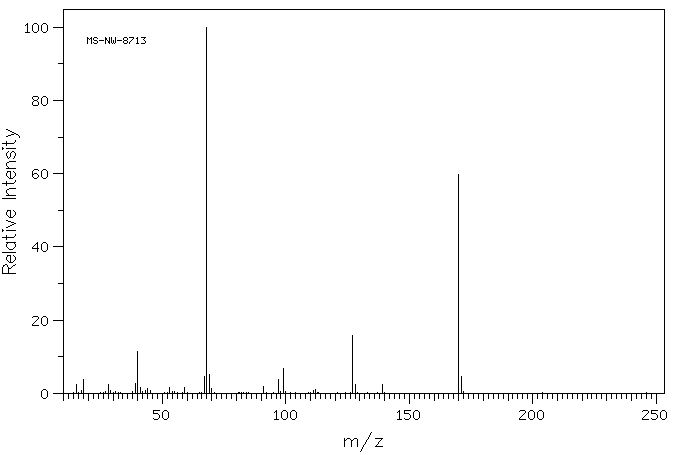
质谱MS
-
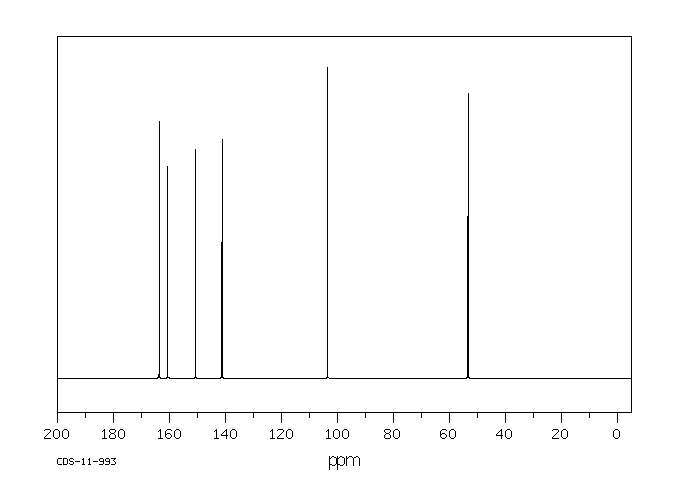
碳谱13CNMR
-
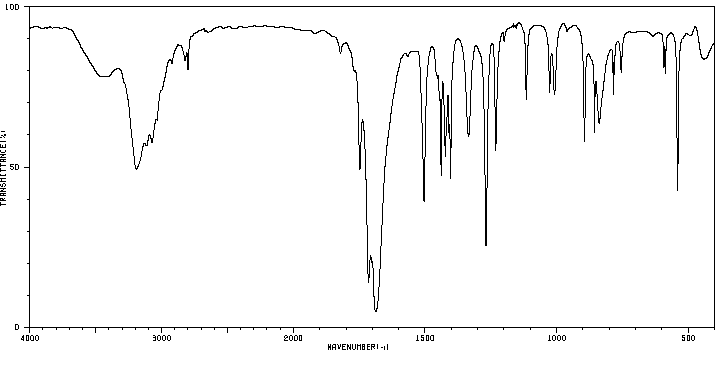
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-3-(2-(二氟甲基)吡啶-4-基)-7-氟-3-(3-(嘧啶-5-基)苯基)-3H-异吲哚-1-胺
(6-羟基嘧啶-4-基)乙酸
(4,5-二甲氧基-1,2,3,6-四氢哒嗪)
鲁匹替丁
马西替坦杂质7
马西替坦杂质4
马西替坦杂质
马西替坦原料药杂质D
马西替坦原料药杂质B
马西替坦
顺式-4-{[5-溴-2-(2,5-二甲基-1H-吡咯-1-基)-6-甲基嘧啶-4-基]氨基}环己醇
非沙比妥
非巴氨酯
非尼啶醇
青鲜素钾盐
雷特格韦钾盐
雷特格韦相关化合物E(USP)
雷特格韦杂质8
雷特格韦EP杂质H
雷特格韦-RT9
雷特格韦
阿西莫司杂质3
阿西莫司
阿脲四水合物
阿脲一水合物
阿维霉素
阿米美啶
阿米洛利
阿米妥钠
阿洛巴比妥
阿普瑞西他滨
阿普比妥
阿巴卡韦相关化合物B(USP)
阿卡明
阿伐那非杂质V
阿伐那非杂质1
阿伐那非杂质
阿伐那非中间体
阿伐那非
铂(2+)二氯化6-甲基-1,3-二{2-[(2-甲基丙基)硫烷基]乙基}嘧啶-2,4(1H,3H)-二酮(1:1)
钴1,2,3,6-四氢-2,6-二氧代嘧啶-4-羧酸酯(1:2)
钠5-烯丙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-乙基-4,6-二氧代-1,4,5,6-四氢-2-嘧啶醇酸酯
钠5-(2-溴丙-2-烯基)-5-丁烷-2-基-4,6-二氧代-1H-嘧啶-2-醇
醌肟腙
酒石酸噻吩嘧啶
那可比妥
辛基2,6-二氧代-1,2,3,6-四氢-4-嘧啶羧酸酯
赛乐西帕杂质3
赛乐西帕KSM3