二乙酰基胺 | 625-77-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75.5-76.5 °C (lit.)
-
沸点:222-223 °C (lit.)
-
密度:1.3846 (rough estimate)
-
溶解度:氯仿(微溶)、甲醇(微溶)
-
蒸汽压力:0.01 mmHg
计算性质
-
辛醇/水分配系数(LogP):-1.4
-
重原子数:7
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:46.2
-
氢给体数:1
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
WGK Germany:3
-
海关编码:2925190090
-
储存条件:请将存放在密封容器内,并置于阴凉、干燥处。
SDS
: 二乙酰基胺
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据化学品全球统一分类与标签制度(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C4H7NO2
分子式
: 101.1 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 75.5 - 76.5 °C - lit.
f) 起始沸点和沸程
222 - 223 °C - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-乙基乙酰胺 N-ethylacetamide 625-50-3 C4H9NO 87.1216 N-乙酰基乙二胺 N-acetylethylenediamine 1001-53-2 C4H10N2O 102.136 —— N-acetylpropionamide 19264-34-7 C5H9NO2 115.132 N-(2-氯乙酰基)乙酰胺 N-acetyl-2-chloroacetamide 17368-73-9 C4H6ClNO2 135.55 —— N-chloro-N-acetylacetamide 59719-20-9 C4H6ClNO2 135.55 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-chloro-N-acetylacetamide 59719-20-9 C4H6ClNO2 135.55
反应信息
-
作为反应物:描述:参考文献:名称:Enzymatic Production Of Peracids Using Perhydrolytic Enzymes摘要:提供了一种工艺,使用至少一种具有过氧化氢酶活性的酶,在中性至酸性反应条件下,利用适当的羧酸酯(包括甘油酯)和/或酰胺底物,在过氧化氢(浓度至少为500 mM)的存在下,原位生产浓缩的水溶性过酸,所得的浓缩过酸溶液足以用于各种消毒和/或漂白应用。公开号:US20090239948A1
-
作为产物:描述:参考文献:名称:Masui, Masaichiro; Hara, Seijiro; Ozaki, Shigeko, Chemical and pharmaceutical bulletin, 1986, vol. 34, # 3, p. 975 - 979摘要:DOI:
-
作为试剂:描述:参考文献:名称:Kocor et al., Bulletin de l'Academie Polonaise des Sciences, Serie des Sciences Chimiques, 1958, vol. 6, p. 1,2摘要:DOI:
文献信息
-
Synthesis of 1,3,5-trisubstituted-1,2,4-triazoles by microwave-assisted N-acylation of amide derivatives and the consecutive reaction with hydrazine hydrochlorides作者:Jongbok Lee、Myengchan Hong、Yoonchul Jung、Eun Jin Cho、Hakjune RheeDOI:10.1016/j.tet.2012.01.003日期:2012.2Facile and efficient procedures for the N-acylation reaction of amide derivatives with various acid anhydrides and the cyclization reaction of N-acylated amide derivatives with various hydrazine hydrochlorides were described. The reactions were carried out under microwave irradiation to give products in good yields in a few minutes. The synthesis of 1,3,5-trisubstituted-1,2,4-triazoles from benzamides
-
Regioselective Three-Component Coupling by the Palladium-Catalyzed Reaction of 2-Fluoroallylic Acetates with Phenols and Imides作者:Motoi Kawatsura、Masaki Kogawa、Hirotaka Watanabe、Mitsuaki Yamamoto、Yukiko Tsuchi、Biao ZhouDOI:10.1055/s-0036-1588143日期:——A palladium-catalyzed three-component coupling reaction of 2-fluoroallylic acetates with phenols, and imides is disclosed. The reaction was effectively catalyzed by [Pd(C3H5)Cl]2/DPPF in the presence of Cs2CO3, and phenols and imides were introduced onto the allyl unit through the C–F bond activation. Furthermore, the reaction proceeds with both high regioselectivity and high Z-selectivity.
-
RhII-Catalyzed Reactions of Diazocarbonyl Compounds with Dicarboximides作者:Vsevolod V. Nikolaev、Heinz Heimgartner、Anthony Linden、Ivan S. Krylov、Valerij A. NikolaevDOI:10.1002/ejoc.200600396日期:2006.10phthalimide and succinimide proceeds chemoselectively at the oxygen atom of the imidic carbonyl group, giving rise to the intermediate formation of carbonyl ylides. Intramolecular stabilization of these highly reactive species occurs in three different ways, and is controlled by the structure of the 2-oxocarbenoids. Carbonyl ylides from diazo esters mainly experience a [1,4]-hydrogen shift, and in this case
-
Si⋯O proximity in imidosilanes – absence of orbital interactions作者:Marcus Herbig、Uwe Böhme、Edwin KrokeDOI:10.1515/znb-2021-0118日期:2022.1.27
Abstract New
N -silylated phthalimides, succinimides, and 1,8-napthalimides were synthesised by reactions of the alkali imides with chlorosilanes in THF. Six different mono-, di-, tri- and tetra-iminosilanes of the type (CH3)4–n Si(imide)n were obtained and the products analysed with1H,13C,29Si NMR, and Raman spectroscopy. The molecular structures of four imidosilanes have been determined by single-crystal X-ray diffraction. A characteristic structural feature of the compounds is the fact that all intramolecular Si⋯O distances are significantly below the sum of the van-der-Waals radii of silicon and oxygen of 3.62 Å. Experimentally found values for Si⋯O distances range from 2.813 to 3.030 Å. However, there are no significant orbital interactions between silicon and oxygen atoms, as shown by quantum chemical analysis with AIM and NBO methods. The short Si⋯O distances in these molecules are caused by the geometry of the rigid imide group bound to the silicon atom, and there is no evidence for an increase of the coordination number of the Si atoms.摘要 通过在四氢呋喃(THF)中将碱金属亚胺与氯硅烷反应,合成了新的N-硅基取代酞酰亚胺、丁二酰亚胺和1,8-萘酰亚胺。得到了六种不同的一、二、三和四亚胺基硅烷,化学式为(CH3)4–
n Si(imide)n ,并使用1H,13C,29Si NMR和拉曼光谱分析了产物。四种亚胺基硅烷的分子结构已通过单晶X射线衍射确定。这些化合物的特征结构特点在于所有分子内的Si⋯O距离显著低于硅和氧的范德华半径之和,即3.62 Å。实验测得的Si⋯O距离范围从2.813到3.030 Å。然而,通过AIM和NBO方法的量子化学分析表明,硅和氧原子之间没有显著的重叠轨道相互作用。这些分子中短的Si⋯O距离是由硅原子连接的刚性的亚胺基团的几何形状造成的,没有证据表明硅原子的配位数增加。 -
Aza spiro compounds acting on the cholinergic system with muscarinic申请人:Israel Institute for Biological Research公开号:US05852029A1公开(公告)日:1998-12-22Compounds useful for treating diseases of the central or peripheral nervous system in mammals have formulae I-XII ##STR1## wherein ring A or A' together with the spiro-carbon atom constitutes a bridged or unbridged ring containing one or two ring nitrogen atoms; and the other symbols have specified values, subject to certain conditions.用于治疗哺乳动物中枢神经系统或外周神经系统疾病的化合物具有以下化学式I-XII ##STR1## 其中环A或A'与螺碳原子一起构成一个含有一个或两个环氮原子的桥接环或非桥接环;其他符号具有特定值,受特定条件约束。
表征谱图
-
氢谱1HNMR
-
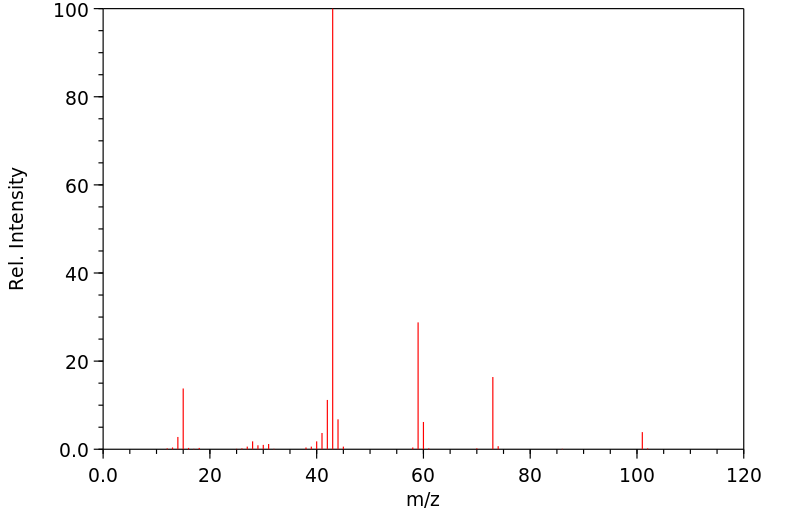
质谱MS
-
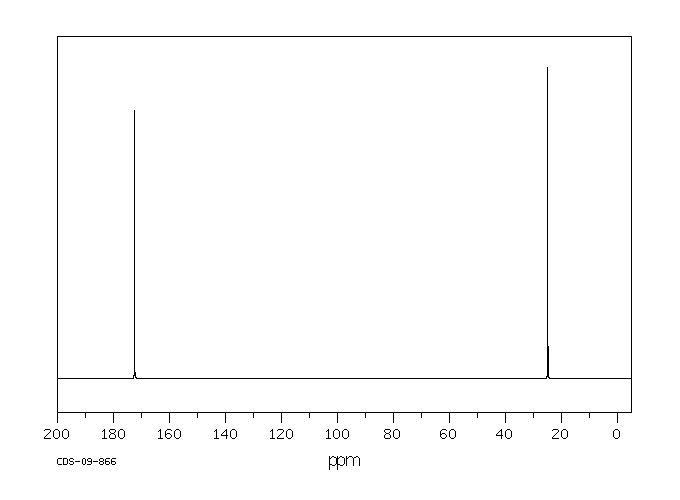
碳谱13CNMR
-
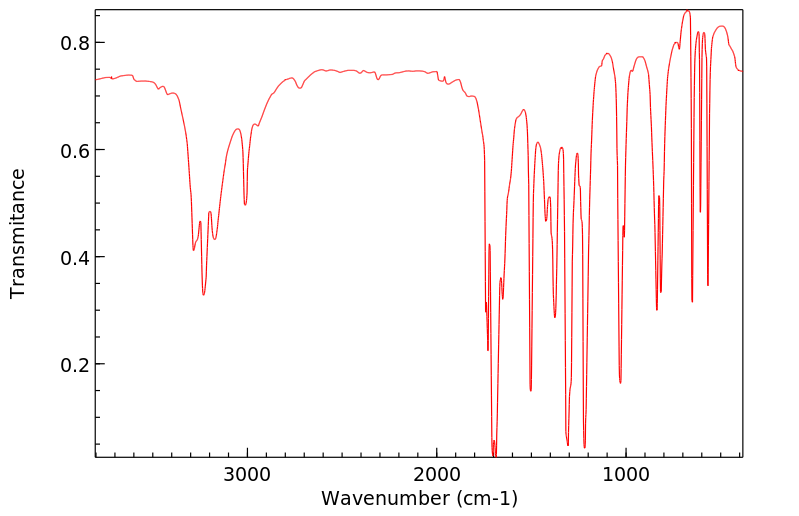
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息