二环己烷并-24-冠醚-8 | 17455-23-1
中文名称
二环己烷并-24-冠醚-8
中文别名
二环己烷并-24-冠-8
英文名称
dicyclohexano-24-crown-8
英文别名
dicyclohexyl-24-crown-8;dicyclohexano-24-crown-8 ether;2,5,8,11,18,21,24,27-octaoxatricyclo[26.4.0.012,17]dotriacontane
CAS
17455-23-1
化学式
C24H44O8
mdl
——
分子量
460.609
InChiKey
QMLGNDFKJAFKGZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:525.41°C (rough estimate)
-
密度:1.102 g/mL at 25 °C(lit.)
-
闪点:>230 °F
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:32
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:73.8
-
氢给体数:0
-
氢受体数:8
安全信息
-
危险等级:6.1(b)
-
危险品标志:T
-
安全说明:S26,S28,S28A,S36/37/39,S38,S45
-
危险类别码:R23/24/25,R36/37/38
-
WGK Germany:3
-
海关编码:2932999099
-
包装等级:III
-
危险类别:6.1(b)
-
危险品运输编号:UN 2810
SDS
| Name: | Dicyclohexano-24-Crown-8 97% Material Safety Data Sheet |
| Synonym: | Dibenz(B,N)(1,4,7,10,13,16,19,22)Octaoxacyclotetracosin, Tetracosahydro- |
| CAS: | 17455-23-1 |
Synonym:Dibenz(B,N)(1,4,7,10,13,16,19,22)Octaoxacyclotetracosin, Tetracosahydro-
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 17455-23-1 | Dicyclohexano-24-Crown-8 | 97 | 241-474-0 |
Risk Phrases: 25
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic if swallowed.Hygroscopic (absorbs moisture from the air).Toxic.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
Harmful if swallowed. May cause irritation of the digestive tract.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Avoid runoff into storm sewers and ditches which lead to waterways.
Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation.
Storage:
Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 17455-23-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: hexane-like
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.1020g/cm3
Molecular Formula: C24H44O8
Molecular Weight: 460.60
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, exposure to moist air or water.
Incompatibilities with Other Materials:
Moisture, strong acids, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 17455-23-1: HO7290000 LD50/LC50:
CAS# 17455-23-1: Draize test, rabbit, eye: 50 mg Moderate; Draize test, rabbit, skin: 100 mg/24H Mild; Oral, rat: LD50 = 75 mg/kg.
Carcinogenicity:
Dicyclohexano-24-Crown-8 - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 25 Toxic if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 17455-23-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 17455-23-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 17455-23-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:二环己烷并-24-冠醚-8 在 fluorine 、 sodium fluoride 作用下, 反应 192.0h, 以3%的产率得到perfluorodicyclohexano-24-crown-8 ether参考文献:名称:全氟大环化合物的合成与化学摘要:全氟大环 perfluoro-18-crown-6, perfluoro-12-crown-4, perfluoro-15-crown-5, perfluoro-cyclohexano-15-crown-5, perfluorodicyclohexano-18-crown-6, perfluorodicyclohexano-24-crown -8, 和 perfluoro-4,7,13,16,21,24-hexaoxa-1, 10-diazabicyclo[8,8,8] hexacosane(perfluorocryptand [222]) 已经通过精心控制的元素氟化制备。尽管它们的碱比它们的烃类似物弱得多,但这些全氟大环是非常稳定的材料,应该有许多应用。报告了全氟-18-冠-6 和全氟二环己基-18-冠-6 异构体的晶体结构。使用几种全氟冠醚和全氟穴状配体 [222] 的气相研究表明,此类大环顽固地结合DOI:10.1021/ja00091a022
-
作为产物:描述:二苯并-24-冠醚-8 以15%的产率得到参考文献:名称:KUSOV, S. Z.;LUBENETS, EH. G.;SEMIKOLENOV, V. A.;KOBRIN, V. S.;XMELNITSKI+, IZV. CO AN CCCP. XIM. N.,(1989) N, S. 62-64摘要:DOI:
-
作为试剂:描述:p-chlorobenzenediazonium tetrafluoroborate 在 二环己烷并-24-冠醚-8 作用下, 以 1,2-二氯乙烷 为溶剂, 生成 alkaline earth salt of/the/ methylsulfuric acid参考文献:名称:与18元,21元和24元冠醚络合的苯二氮杂离子的去重氮率摘要:在18-crown-6、21-crown-7和dicyclohexano-24-crown-8存在下,在50°C下于1,2-二氯乙烷中对四氟硼酸苯重氮鎓和四氟硼酸-氯苯重氮鎓进行重氮化的动力学评价表明,对于具有21冠7的配合物,复合物中去重氮的速率常数最小,而形成复合物的平衡常数最大。DOI:10.1016/s0040-4039(01)81825-6
文献信息
-
Sequential <i>O</i>- and <i>N</i>-Acylation Protocol for High-Yield Preparation and Modification of Rotaxanes: Synthesis, Functionalization, Structure, and Intercomponent Interaction of Rotaxanes作者:Yuya Tachibana、Hiroaki Kawasaki、Nobuhiro Kihara、Toshikazu TakataDOI:10.1021/jo0601563日期:2006.7.1catalyst to afford the corresponding rotaxane in high yield. Large-scale synthesis without chromatographic separation was easily achieved. The ammonium group in the resulting rotaxane was quantitatively acylated with excess electrophile in the presence of excess trialkylamine. Various N-functionalized rotaxanes were prepared by this sequential double-acylation protocol. 1H NMR spectra and X-ray crystallographic在三丁基膦作为催化剂的存在下,由庞大的酸酐将由24元冠醚和在末端带有羟基的仲铵盐组成的拟轮烷定量酰化,从而以高收率得到相应的轮烷。无需色谱分离即可轻松实现大规模合成。在过量的三烷基胺存在下,用过量的亲电子试剂将所得轮烷中的铵基团定量酰化。通过该顺序的双酰化方案制备了各种N-官能化的轮烷。1个轮烷的1 H NMR光谱和X射线晶体学分析表明,冠状醚组分通过强的氢键键合相互作用而被捕获在铵型轮烷中的铵基上。铵基周围的构象通过氢键相互作用而固定。同时,酰胺型轮烷的构象由冠醚中的亚甲基与车轴组件的苯环之间的弱CH /π相互作用确定。铵型轮烷的N-酰化可用于制备功能化的轮烷和弱的基于组分间相互作用的轮烷。
-
Efficient synthesis of [2]- and higher order rotaxanes via the transition metal-catalyzed hydrosilylation of alkyne作者:Hisahiro Sasabe、Nobuhiro Kihara、Kazuhiko Mizuno、Akiya Ogawa、Toshikazu TakataDOI:10.1016/j.tetlet.2005.03.186日期:2005.5RuHCl(CO)(PPh3)3 and RhCl(CO)(PPh3)3 complexes catalyzed the hydrosilylation reactions of the alkyne moiety of several pseudorotaxanes at ambient temperature to give the corresponding [2]- and higher order rotaxanes in high yields with excellent regio- and stereoselectivity.
-
Dynamically capped rotaxanes: metal coordination vs. acid–base pairing in the chiral end-capping作者:Satoshi Shinoda、Takayuki Maeda、Hiroyuki Miyake、Hiroshi TsukubeDOI:10.1080/10610278.2010.521838日期:2011.3.1formed by mixing a thread molecule and several crown ethers. Crystal structure analysis clearly indicated that hydrogen bonding between NH2 + of the thread molecule and crown ether oxygen atoms was significantly involved, together with cation–dipole interactions between cationic nitrogen and ether oxygen atoms. End-capping of the pseudorotaxane with chiral metal complexes gave dynamically capped rotaxanes
-
New concept for the preparation of potassium sodides: the use of hexane as a non-polar solvent作者:Zbigniew Grobelny、Andrzej Stolarzewicz、Barbara Morejko-Buż、Antoni WiniarskiDOI:10.1016/s0040-4020(03)00185-6日期:2003.3contact with 15-crown-5 hexane solution became potassium sodide K+(15-crown-5)2Na−. After the evaporation of hexane the crystalline solid product was analyzed by X-ray diffraction and the lattice parameters were calculated. The potassium sodide thus obtained could be easily dissolved in tetrahydrofuran. A deep blue solution containing sodium anions and complexed potassium cations was formed with a very
-
Dry optical - chemical carbon - dioxide sensor申请人:F. Hoffmann-La Roche AG公开号:EP1972929A1公开(公告)日:2008-09-24A device for determining carbon dioxide in a gaseous or liquid sample, comprises: a polymer matrix e.g. ethyl cellulose, polysiloxanes etc. and an indicator embedded in the polymer matrix, wherein the indicator comprises a pH-sensitive dye e.g. bromothymol blue, HPTS etc. and a lipophilic, metal cation-ionophore complex e.g. K-18-crown-6, and wherein an anion of the pH-sensitive dye and the metal cation-ionophore complex form an ion-pair which is soluble in the polymer matrix.
表征谱图
-
氢谱1HNMR
-
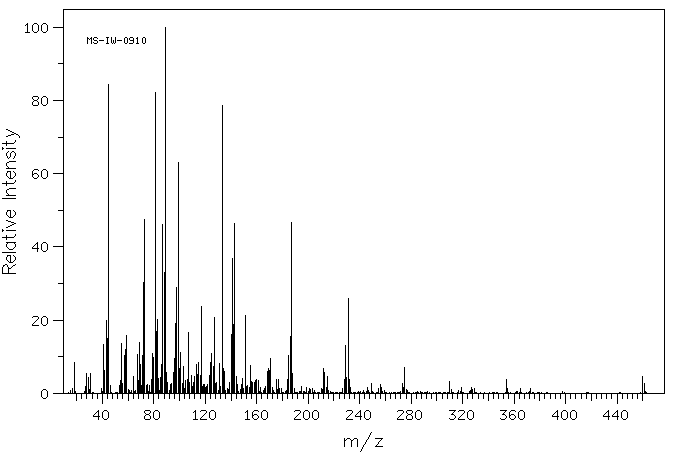
质谱MS
-
碳谱13CNMR
-
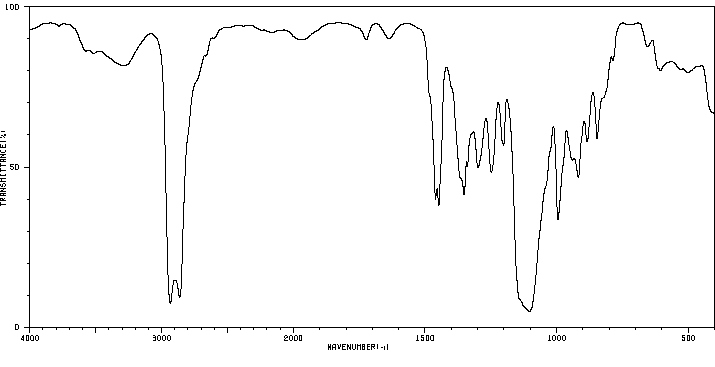
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷