依替前列通 | 565-63-9
中文名称
依替前列通
中文别名
2-甲基异巴豆酸;(Z)-2-甲基-2-丁烯酸;当归酸;白芷酸;2-甲基-2-丁烯酸;欧白芷酸;顺式-2,3-二甲基丙烯酸;顺式-2-甲基丁烯酸
英文名称
Angelic acid
英文别名
(Z)-2-methyl-2-butenoic acid;2,3-Dimethylacrylic acid, (Z)-;(Z)-2-methylbut-2-enoic acid
CAS
565-63-9
化学式
C5H8O2
mdl
——
分子量
100.117
InChiKey
UIERETOOQGIECD-ARJAWSKDSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:44°C
-
沸点:96 °C / 12mmHg
-
密度:1.010
-
闪点:96℃
-
溶解度:在甲醇中几乎透明
-
LogP:1.076 (est)
-
物理描述:Solid
-
保留指数:919.7;898
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:7
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:8
-
安全说明:S26,S36/37/39
-
危险类别码:R34
-
海关编码:2916190090
-
危险品运输编号:UN 3261
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P501,P260,P234,P264,P280,P390,P303+P361+P353,P301+P330+P331,P363,P304+P340+P310,P305+P351+P338+P310,P406,P405
-
危险性描述:H314,H290
-
储存条件:本品应密封存放在干燥、避光的地方。
SDS
当归酸 修改号码:6
模块 1. 化学品
产品名称: Angelic Acid
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
当归酸 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 当归酸
百分比: >98.0%(GC)(T)
CAS编码: 565-63-9
俗名: (Z)-2-Methyl-2-butenoic Acid , 2-Methylisocrotonic Acid
分子式: C5H8O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
当归酸 修改号码:6
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 44°C
沸点/沸程 96 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
当归酸 修改号码:6
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 3261
正式运输名称: 腐蚀性固体, 酸性的, 有机的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Angelic Acid
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
金属腐蚀性 第1级
健康危害
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 可能腐蚀金属
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 只可存放于原用的容器内。
切勿吸入。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:漱口。切勿催吐。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
吸收溢出物,防止材料被损坏。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
当归酸 修改号码:6
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 当归酸
百分比: >98.0%(GC)(T)
CAS编码: 565-63-9
俗名: (Z)-2-Methyl-2-butenoic Acid , 2-Methylisocrotonic Acid
分子式: C5H8O2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。切勿引吐。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
使用耐腐蚀设备。
贮存
储存条件: 保持容器密闭。冷藏储存。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。只可存放在原用的容器內。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
当归酸 修改号码:6
模块 8. 接触控制和个体防护
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 44°C
沸点/沸程 96 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
当归酸 修改号码:6
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
UN编号: 3261
正式运输名称: 腐蚀性固体, 酸性的, 有机的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
生物活性
Angelica Acid 存在于马郁兰精油中,以酯的形式存在。这种成分有助于伤口愈合,并具备镇静和精神方面的特性。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 惕格酸 Tiglic acid 80-59-1 C5H8O2 100.117 当归酸甲酯 methyl angelate 5953-76-4 C6H10O2 114.144 3-溴-2-甲基丁-2-烯酸 (E)-3-bromo-2-methylbut-2-enoic acid 35057-99-9 C5H7BrO2 179.013 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 惕格酸 Tiglic acid 80-59-1 C5H8O2 100.117 当归酸甲酯 methyl angelate 5953-76-4 C6H10O2 114.144 惕各酸乙酯 Ethyl tiglate 5837-78-5 C7H12O2 128.171
反应信息
-
作为反应物:参考文献:名称:Demarcay, Chemische Berichte, 1876, vol. 9, p. 1933摘要:DOI:
-
作为产物:描述:2-羟基-2-甲基丁酸 生成 依替前列通参考文献:名称:Blaise; Bagard, Annales de Chimie (Cachan, France), 1907, vol. <8>11, p. 116摘要:DOI:
文献信息
-
Asymmetric Total Synthesis of the Indole Diterpene Alkaloid Paspaline作者:Robert J. Sharpe、Jeffrey S. JohnsonDOI:10.1021/acs.joc.5b01844日期:2015.10.2An enantioselective synthesis of the indole diterpenoid natural product paspaline is disclosed. Critical to this approach was the implementation of stereoselective desymmetrization reactions to assemble key stereocenters of the molecule. The design and execution of these tactics are described in detail, and a thorough analysis of observed outcomes is presented, ultimately providing the title compound
-
Sodium Methyl Carbonate as an Effective C1 Synthon. Synthesis of Carboxylic Acids, Benzophenones, and Unsymmetrical Ketones作者:Timothy E. Hurst、Julie A. Deichert、Lucas Kapeniak、Roland Lee、Jesse Harris、Philip G. Jessop、Victor SnieckusDOI:10.1021/acs.orglett.9b00773日期:2019.6.7Reported is the synthesis of carboxylic acids, symmetrical ketones, and unsymmetrical ketones with selectivity achieved by exploiting the differential reactivity of sodium methyl carbonate with Grignard and organolithium reagents.
-
Laserine oxide, an epoxide from Guillonea scabra作者:Mariano Pinar、Manuel Rico、Benjamín RodríguezDOI:10.1016/0031-9422(82)83177-4日期:——Abstract From the roots of Guillonea scabra several previously known compounds were isolated. In addition, a new epoxy derivative of laserine was obtained and its structure was established by chemical and spectroscopic means.
-
Asymmetric Dihydroxylation of Esters and Amides of Methacrylic, Tiglic, and Angelic Acid: No Exception to the Sharpless Mnemonic!作者:Fabian Weber、Reinhard BrücknerDOI:10.1002/ejoc.201403622日期:2015.4are contradictory and partly in conflict with the Sharpless mnemonic. We systematically screened the SAD of esters and amides of angelic, tiglic, and methacrylic acid. Enantiocontrol arose in 14 of the 15 reactions, culminating at 99 % ee for the Weinreb amide of tiglic acid. The absolute configurations of all the nonracemic products were established by (stereo)chemical correlations. Without exception
-
First Comprehensive Bakkane Approach: Stereoselective and Efficient Dichloroketene-Based Total Syntheses of (±)- and (−)-9-Acetoxyfukinanolide, (±)- and (+)-Bakkenolide A, (−)-Bakkenolides III, B, C, H, L, V, and X, (±)- and (−)-Homogynolide A, (±)-Homogynolide B, and (±)-Palmosalide C作者:Timothy J. Brocksom、Fernando Coelho、Jean-Pierre Deprés、Andrew E. Greene、Marco E. Freire de Lima、Olivier Hamelin、Benoît Hartmann、Alice M. Kanazawa、Yanyun WangDOI:10.1021/ja0208456日期:2002.12.1dichloroketene with dimethylcyclohexenes has been used as the key reaction in an efficient, general approach to the bakkanes. New methods and methodologies that have been developed in this work include spiro beta-methylene-gamma-butyrolactonizations, a vicinal dicarboxylation, an angelic ester preparation, a transesterification, an epoxy ketone double reduction, and a retro aldol-aldol approach to low-energy aldol
表征谱图
-
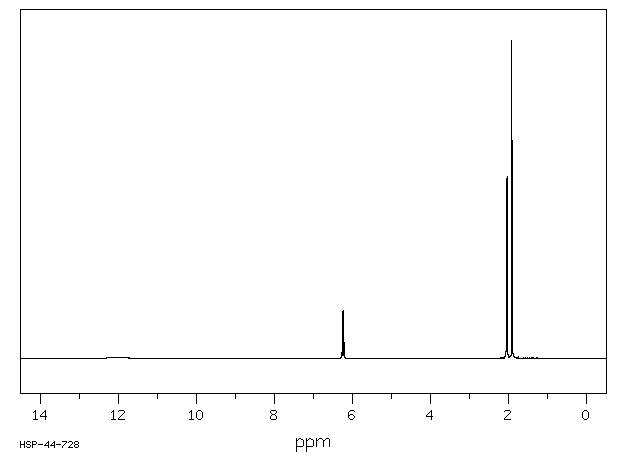
氢谱1HNMR
-
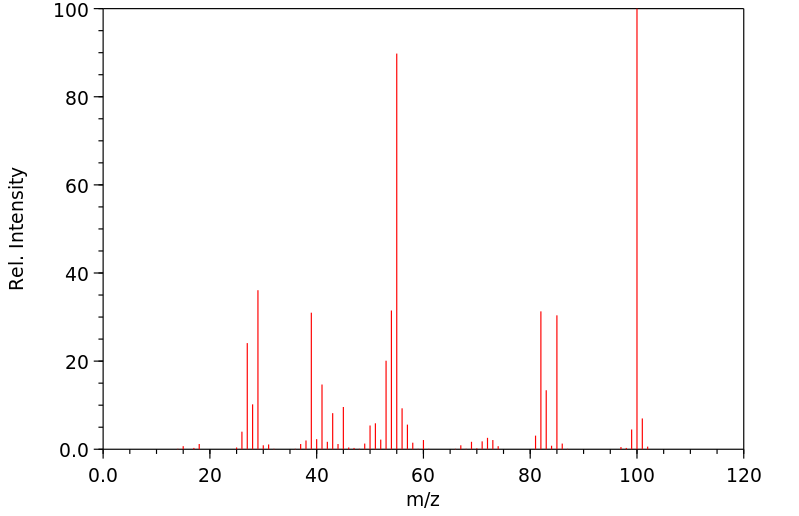
质谱MS
-
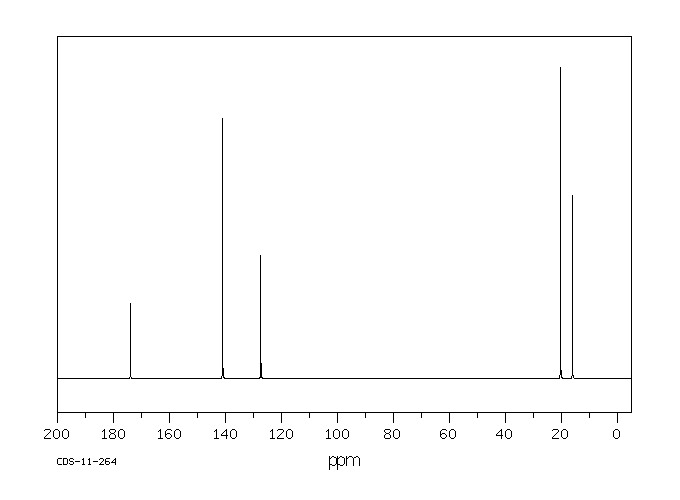
碳谱13CNMR
-
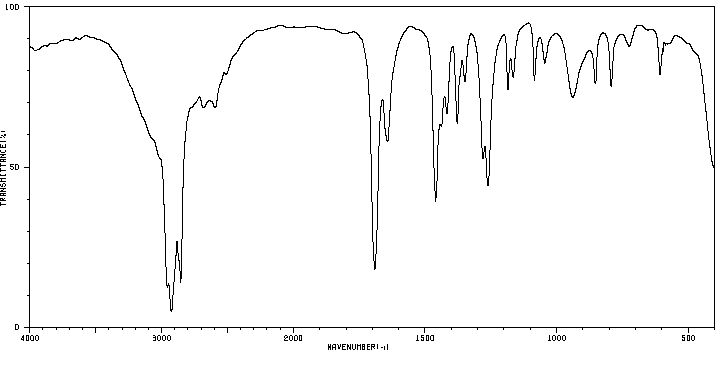
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(±)17,18-二HETE
(±)-辛酰肉碱氯化物
(Z)-5-辛烯甲酯
(Z)-4-辛烯酸
(R)-甲羟戊酸锂盐
(R)-普鲁前列素,游离酸
(R,R)-半乳糖苷
(E)-4-庚烯酸
(E)-4-壬烯酸
(E)-4-十一烯酸
(9Z,12E)-十八烷二烯酸甲酯
(6E)-8-甲基--6-壬烯酸甲基酯-d3
(3R,6S)-rel-8-[2-(3-呋喃基)-1,3-二氧戊环-2-基]-3-羟基-2,6-二甲基-4-辛酮
龙胆二糖
黑曲霉二糖
黄质霉素
麦芽酮糖一水合物
麦芽糖醇
麦芽糖酸
麦芽糖基蔗糖
麦芽糖一水合物
麦芽糖
鳄梨油酸乙酯
鲸蜡醇蓖麻油酸酯
鲸蜡醇油酸酯
鲸蜡硬脂醇硬脂酸酯
鲸蜡烯酸脂
鲸蜡基花生醇
鲫鱼酸
鲁比前列素
鲁比前列素
高级烷基C16-18-醇
高甲羟戊酸
高效氯氰菊酯
高-gamma-亚油酸
马来酸烯丙酯
马来酸氢异丙酯
马来酸氢异丁酯
马来酸氢丙酯
马来酸氢1-[2-(2-羟基乙氧基)乙基]酯
马来酸单乙酯
马来酸单丁酯
马来酸二辛酯
马来酸二癸酯
马来酸二甲酯
马来酸二烯丙酯
马来酸二正丙酯
马来酸二戊基酯
马来酸二异壬酯
马来酸二异丙酯