双(2,4,5-三氯苯基)二硫化物 | 3808-87-5
中文名称
双(2,4,5-三氯苯基)二硫化物
中文别名
双(2,4,5-三氯苯基)二硫醚;双(2,4,6-三氯苯基)二硫化物;2,4,5-三氯苯基二硫醚;二(2,4,5-三氯苯)二硫醚;二(2,4,5-三氯苯基)二硫醚
英文名称
2,4,5-trichlorophenyl disulfide
英文别名
bis(2,4,5-trichlorophenyl) disulfide;1,2,4-trichloro-5-[(2,4,5-trichlorophenyl)disulfanyl]benzene
CAS
3808-87-5
化学式
C12H4Cl6S2
mdl
MFCD00000551
分子量
425.014
InChiKey
ZUVJVEOHQKNMPN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140-144 °C(lit.)
-
沸点:460.9±45.0 °C(Predicted)
-
密度:1.6505 (estimate)
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):7.8
-
重原子数:20
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:50.6
-
氢给体数:0
-
氢受体数:2
安全信息
-
WGK Germany:3
-
安全说明:S24/25
-
海关编码:2930909090
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | 2 4 5-Trichlorophenyl Disulfide 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 3808-87-5 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3808-87-5 | 2,4,5-Trichlorophenyl Disulfide | 98% | 223-279-2 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 3808-87-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 140.00 - 144.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
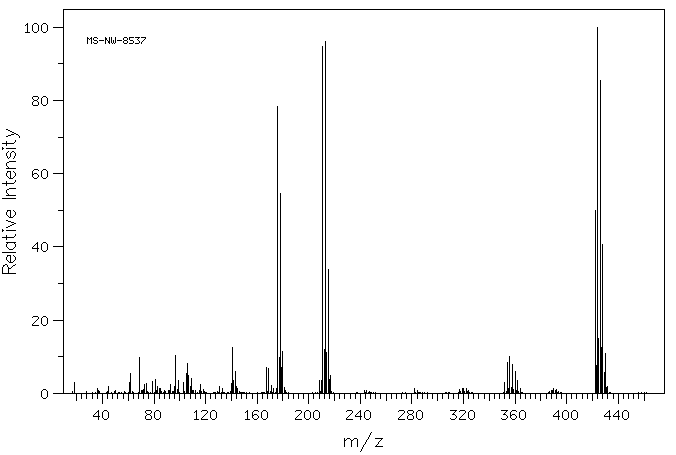
Molecular Formula: C12H4Cl6S2
Molecular Weight: 425.00
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents, strong bases.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3808-87-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2,4,5-Trichlorophenyl Disulfide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 3808-87-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3808-87-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3808-87-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
染料和橡胶的制备。
用途简介暂无相关内容。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— bis-(2,4,5-trichloro-phenyl)-trisulfide 24082-44-8 C12H4Cl6S3 457.08 2,4,5-三氯苯硫醇 2,4,5-trichlorothiophenol 3773-14-6 C6H3Cl3S 213.515 —— 2,4,5-trichlorobenzenesulfenyl chloride 62726-90-3 C6H2Cl4S 247.96 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4,5-三氯苯硫醇 2,4,5-trichlorothiophenol 3773-14-6 C6H3Cl3S 213.515 2,4,5-三氯硫代苯甲醚 2,4,5-trichloromethylthiobenzene 4163-78-4 C7H5Cl3S 227.542 —— 2,4,5-trichlorobenzenesulfenyl chloride 62726-90-3 C6H2Cl4S 247.96
反应信息
-
作为反应物:描述:参考文献:名称:Reduction of Sulfonic Acids with Triphenylphosphine–Diaryl Disulfide System摘要:二芳基二硫化合物是有效的催化剂,能与三苯基膦共用,将芳基磺酸还原为相应产率良好的芳基硫醇,同时将烷基磺酸转化为相应的烷基芳基硫醚。电子供体取代的芳基磺酸比电子受体取代的芳基磺酸更容易被还原,而具有电子受体取代基的二芳基二硫化合物比具有电子供体环取代基的二芳基二硫化合物更有效作为催化剂。DOI:10.1246/bcsj.57.232
-
作为产物:描述:参考文献:名称:612.一些芳磺酰氯的反应摘要:DOI:10.1039/jr9600003063
文献信息
-
A simple and highly effective oxidative chlorination protocol for the preparation of arenesulfonyl chlorides作者:Yu-Ming Pu、Alan Christesen、Yi-Yin KuDOI:10.1016/j.tetlet.2009.11.047日期:2010.1suitable for many types of sulfur substrates (thiols, disulfides, and benzylic sulfides). The overall process is simple, practical, and it provides convenient access to a variety of aryl or heteroarylsulfonyl chlorides. The mild reaction conditions and the broad substrate scope render this method attractive and complementary to existing syntheses of aryl or heteroarylsulfonyl chlorides.
-
Clay-supported reagents IV. A novel coupling of thiols into disulphides, via thionitrite intermediates using a clay-supported nitrosation reagent.
-
A Novel Oxidizing Reagent Based on Potassium Ferrate(VI)<sup>1</sup>作者:Lionel Delaude、Pierre LaszloDOI:10.1021/jo960633p日期:1996.1.1overoxidation to benzoic acid. The roles of the various parameters (reaction time and temperature, nature of the solvent, method of preparation of the solid reagent) were investigated. The evidence points to a polar reaction mechanism. The ensuing procedure was applied successfully, at room temperature, to oxidation of a series of alcohols to aldehydes and ketones, to oxidative coupling of thiols to一种新的,有效的准备已设计出高铁酸钾(VI)(K(2)FeO(4))。研究了这种高价铁盐在非水介质中氧化有机底物的能力。以苯甲醇为模型,确定了多种微孔吸附剂的催化活性。在众多的铝硅酸盐类型的固体载体中,发现K10蒙脱石粘土最能实现苯甲醛的定量形成,而不会过度氧化为苯甲酸。研究了各种参数(反应时间和温度,溶剂的性质,固体试剂的制备方法)的作用。有证据表明存在极性反应机制。随后的程序在室温下成功地用于将一系列醇氧化为醛和酮,硫醇与二硫化物的氧化偶联以及氮衍生物的氧化。在75摄氏度下,该试剂具有氧化活性烃和非活性烃的能力。甲苯变成苯甲醇(和苯甲醛)。环烷烃也以显着(30-40%)的产率被氧化成各自的环烷醇(和环烷酮)。因此,与适当的多相催化剂一起使用的高铁酸钾是一种强力且对环境友好的氧化剂。到相应的环烷醇(和环烷酮)。因此,与适当的多相催化剂一起使用的高铁酸钾是一种强力且对环境友好的氧化剂。到相
-
Sulfenylation of heterocyclic 1,3-dicarbonyl systems: 4-Hydroxy-2-pyrones, 6-hydroxy-4-pyrimidones, 4-hydroxy-2-pyridones, 4-hydroxy-6-pyridazinones, and 5-hydroxy-3-pyrazolones作者:Barbara Schnell、Thomas KappeDOI:10.1002/jhet.5570370439日期:2000.7compounds 1, 9,14, and 19, react in dimethylformamide in the presence of potassium carbonate with diaryl disulfides 2 to yield arylsulfenyl derivatives (3, 10, 15, 20). The arylthiolate anions 4 formed in this reaction can be oxidized by air to yield the starting disulfides 2 again. Tetraalkylthiuram disulfides 7 react in the same manner to yield dialkylaminothiocarbonylthio derivatives (8, 13, 18) of the title
-
Chemoselective C-S/S-S Formation between Diaryl Disulfides and Tetraalkylthiuram Disulfides作者:Kang Peng、Hui Zhu、Xing Liu、Han-Ying Peng、Jin-Quan Chen、Zhi-Bing DongDOI:10.1002/ejoc.201901401日期:2019.12.19An efficient C–S/S–S formation for the chemoselective synthesis of aryl dithiocarbamate (C–S formation) and aryl dialkylcarbamo(dithioperoxo)thioate (S–S formation) was studied. The transformation is simple and features easily available starting materials, high selectivity, showing its potential to synthesize biologically or pharmaceutically active compounds.
表征谱图
-
氢谱1HNMR
-
质谱MS
-
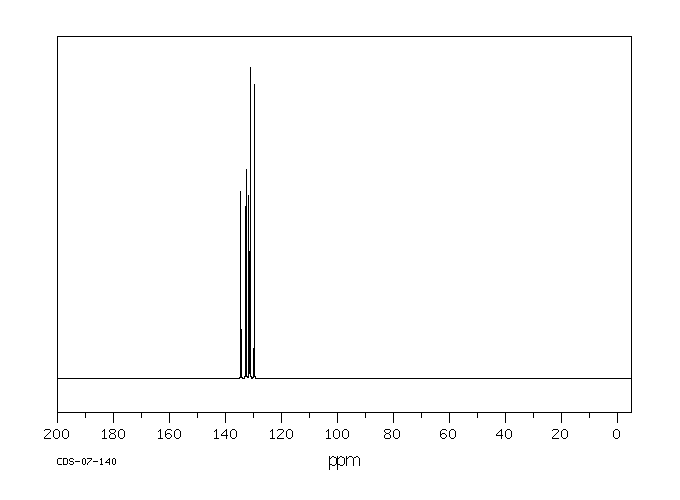
碳谱13CNMR
-
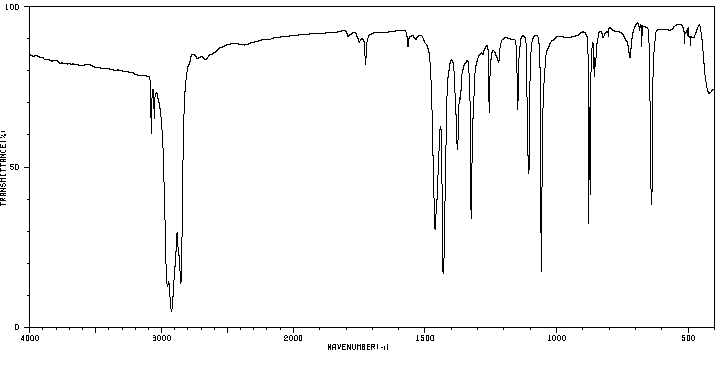
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫